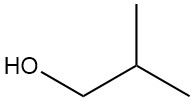
2-Methyl-1-propanol, also known as isobutanol or isobutyl alcohol, is an organic compound with the chemical formula (CH3)2CHCH2OH. It is a colorless liquid with a characteristic smell that occurs in natural products as well as in fusel oils.
Isobutanol is a product of molasses fermentation (comprising approximately 74% of the total alcohol content). Historically, isobutanol was isolated from fusel oils. However, the development of the isobutyl oil synthesis, a process analogous to methanol synthesis from carbon monoxide and hydrogen, enabled the large-scale production.
In the 2010s, a significant shift had occured with the propylene hydroformylation (oxo synthesis) becoming the current dominant production method due to its efficiency and cost-effectiveness. This has led to a significant increase in isobutanol market size.
Table of Contents
1. Physical Properties of 2-Methyl-1-propanol
2-Methyl-1-propanol (isobutanol) is a colorless liquid with a characteristic odor. Its vapors are irritant to mucous membranes and can have a narcotic effect at high concentrations. This alcohol is miscible with common organic solvents.
Table 1 summarizes the key physical properties of 2-methyl-1-propanol:
| Property | Value |
|---|---|
| Molar mass | 74.12 g/mol |
| Melting point (mp) | -107.9 °C |
| Boiling point (bp) | 107.9 °C |
| Density at 20°C (d20) | 0.8027 g/mL |
| Refractive index at 20°C (n20) | 1.3959 |
| Viscosity at 20°C | 4.0 mPa·s |
| Specific heat (30-80°C) | 2.5263 J·g-1·K-1 |
| Heat of vaporization | 578.83 J/g |
| Heat of combustion | 35.981 kJ/g |
| Critical pressure | 48 hPa |
| Critical temperature | 265 °C |
| Surface tension (room temp.) | 23.0 mN/m |
| Dielectric constant (room temp.) | 18.8 |
| Evaporation number (ether = 1) | 24 |
| Solubility in water |
20°C: 8.5 wt% 30°C: 7.5 wt% |
| Solubility of water in isobutanol |
20°C: 15 wt% 30°C: 17.3 wt% |
| Flash point | 28 °C |
| Ignition limits in air | 1.7-10.9 vol% |
| Ignition temperature | 430 °C |
2. Chemical Properties of 2-Methyl-1-propanol
2.1. Dehydration
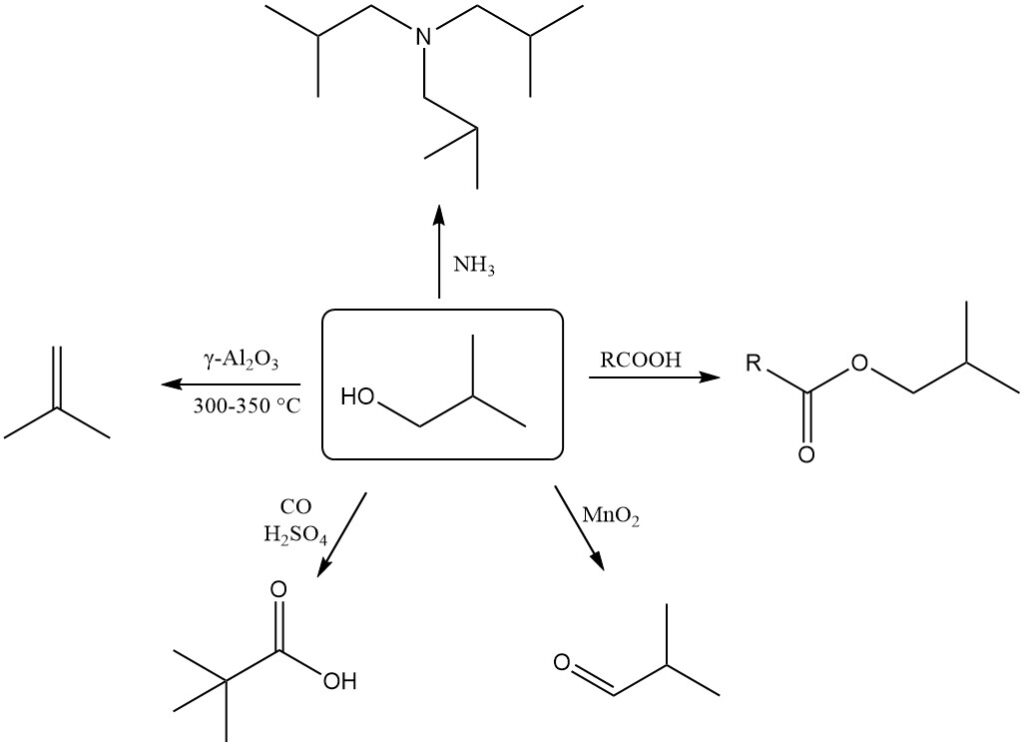
2-Methyl-1-propanol readily undergoes dehydration to form 2-methylpropene (isobutene). This reaction requires γ-Al2O3 catalyst at 300-350°C and achieves nearly quantitative conversion with over 90% selectivity for isobutene.
2.2. Oxidation
2-Methyl-1-propanol can be dehydrogenated to 2-methylpropanal (isobutanal) using oxidizing agents like manganese dioxide or suitable catalysts at higher temperatures.
The Koch-Haaf reaction converts 2-methyl-1-propanol to trimethylacetic acid in the presence of carbon monoxide and sulfuric acid with a high yield (89%).
Oxidation of isobutanol with dichromate in the presence of sulfuric acid produces isobutyric acid.
2.3. Alkylation
2-Methyl-1-propanol can be used for alkylation reactions with ammonia or amines to form various alkylamines.
2.4. Esterification
2-Methyl-1-propanol reacts with inorganic and organic acids in the presence of catalysts to form isobutyl esters, as well as with acid chlorides and acid anhydrides.
3. Production of 2-Methyl-1-propanol
Three important industrial processes for producing 2-methyl-1-propanol (isobutanol) are as follow:
- Propene hydroformylation (oxo synthesis)
- Catalytic hydrogenation of carbon monoxide
- Homologization reaction
3.1. Propene hydroformylation (oxo synthesis)
The oxo synthesis is the most efficient and cost-effective method for large-scale production of isobutanol. It involves reacting propene with synthesis gas (a mixture of carbon monoxide and hydrogen) under high pressure and temperature, using a suitable catalyst like rhodium or cobalt carbonyl complexes.
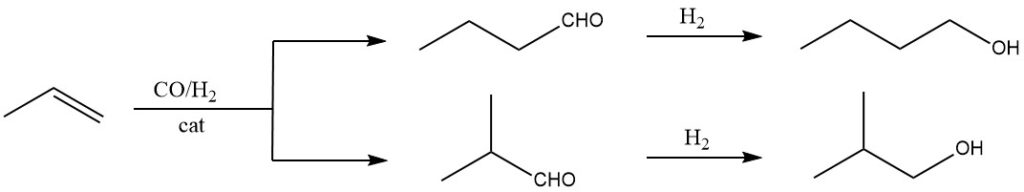
It form a mixture of alcohols (1-butanol and 2-methyl-1-propanol). This is currently the dominant method for the synthesis of isobutanol due to its high efficiency, cost-effectiveness, and readily available starting materials.
For more information, consult the article about 1-butanol.
3.2. Catalytic Hydrogenation of Carbon Monoxide
While the CO-H2-based methanol synthesis achieved commercial success in the 1920s, analogous processes for higher alcohols faced limited adoption. Despite initial promise, these processes have largely disappeared from the industrial landscape.
Among these historical attempts, the BASF isobutanol oil synthesis holds particular significance. This process utilized a KOH-modified methanol synthesis catalyst under high pressure (30 MPa) and elevated temperature (430 °C) to convert CO and H2 into a product mixture containing roughly 50% methanol, 11-14% 2-methyl-1-propanol (isobutanol), and other components.
However, BASF abandoned the isobutanol oil synthesis in 1952 as more economical alternatives emerged. The oxo synthesis and petrochemical routes offered superior efficiency and cost-effectiveness for both isobutanol and its valuable secondary product, 2-methylpropene.
3.3. Homologization reaction
This process involves extending the carbon chain of a shorter alcohol such as methanol or ethanol or alkane to produce isobutanol. While technically possible, suitable homologization reactions for isobutanol production are not very efficient or commercially viable compared to propene hydroformylation.
Attempts to achieve direct butanol production from readily available methanol and ethanol via homologization reactions have not yielded significant results. Despite extensive research efforts, isobutanol have only been obtained in minor quantities as byproduct.
Other processes include the hydration of 2-methylpropene with 65% sulfuric acid to form isobutanol.

4. Uses of 2-Methyl-1-Propanol
2-Methyl-1-propanol is often compared to 1-butanol due to their similar applications and finds use in various industries due to its lower cost and unique properties.
Solvents and Additives:
- Cost-effective substitute for 1-butanol: 2-methyl-1-propanol serves as a solvent, diluent, and additive in nitrocellulose and synthetic resins, cleaning agents, and printing inks.
- Resin compatibility: It is able to dissolve ketone resins, phthalate resins, urea resins, and melamine-formaldehyde resins and is used commercially, although it is less effective as a solvent for phenol-formaldehyde resins compared to 1-butanol.
Esters:
- Isobutyl acetate: This ester is used as a solvent for fats, chlorinated rubber, polystyrene, and coumarone resins.
- Plasticizers: Phthalic, adipic, and dicarboxylic acid esters, as well as the phosphoric acid ester of 2-methyl-1-propanol, find use as plasticizers, particularly for PVC and its copolymers and for cellulose derivatives.
- Chlorophenoxyacetic acid esters: The isobutyl esters of 2,4-dichloro- and 2,4,5-trichlorophenoxyacetic acids have herbicidal activity.
Other Applications:
- Gasoline anti-freeze: 2-methyl-1-propanol possesses anti-freezing properties in gasoline.
- Ammonium phosphate extraction: Its use as an extraction agent in ammonium phosphate recovery adds to its versatility.
5. Toxicology of 2-Methyl-1-Propanol
2-Methyl-1-propanol shows low acute oral and dermal toxicity but moderate aquatic toxicity. Effects include eye and skin irritation.
Acute Toxicity:
- Low oral toxicity: LD50 in rats is 2460 mg/kg (lowest published).
- Low dermal toxicity: LD50 in rabbits is 3400 mg/kg.
- Moderate aquatic toxicity: LC50 for fish is 1330 mg/L (96h).
- Narcosis observed in mice at high inhalation exposure (6400 ppm).
Local Effects:
- Moderate to severe eye irritation in rabbits.
- Moderate skin irritation in rabbits (24h exposure).
Chronic Effects:
- Long-term studies suggest possible carcinogenicity in rats (limited data).
- Mutagenic in E. coli bacteria.
Human Exposure:
- No eye irritation observed in humans at low inhalation exposure (100 ppm).
- Mixed vapor exposure (butyl acetate and 2-methyl-1-propanol) caused vacuolar keratitis in workers (causative agent unclear).
- Skin application caused slight erythema and hyperemia in humans.
Exposure Limits:
- TLV (Threshold Limit Value): 50 ppm (TWA), 75 ppm (STEL) [ACGIH]
- MAK (Maximum Allowable Concentration): 100 mL/m³ [Germany]
- TRGS 900 (Occupational Exposure Limit): 100 mL/m³ [Germany]
Reference
- Butanols; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a04_463.pub3


