Pyruvic Acid: Properties, Reactions, Production and Uses
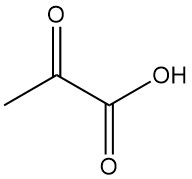
What is Pyruvic Acid?
Pyruvic acid, also known as 2-oxopropanoic acid, pyroracemic acid, or α-ketopropionic acid, is an important α-oxocarboxylic acid with the formula C3H4O3. It is a colorless liquid with an odor similar to that of acetic acid that plays an important role in energy metabolism in living organisms.
Pyruvic acid is synthesized from glycogen within muscle tissue during periods of exertion and subsequently reduced to lactic acid. The hepatic metabolism of pyruvic acid to alanine occurs via reductive amination.
The first discovery and description of pyruvic acid is attributed to Berzelius in 1835.
Table of Contents
1. Physical Properties of Pyruvic Acid
Pyruvic acid is a colorless liquid with an odor like sour vinegar. It is miscible with water, ethanol, and ether and exists only in the keto form; the enol form has not been detected.
The most important physical properties of pyruvic acid are summarized in the following table.
| Property | Value |
|---|---|
| CAS number | [127-17-3] |
| Chemical formula | H3C-CO-COOH |
| Molecular weight | 88.06 g/mol |
| Melting point | 13.6 °C |
| Boiling point | at 101.31 kPa: 165 °C (decomposition) at 1.33 kPa: 57.9 °C at 0.13 kPa: 21.4 °C |
| Density (20 °C) | 1.268 g/cm3 |
| Refractive index (25 °C) | 1.4259 |
| pKa | 2.45 (at 25 °C) |
| Vapor Pressure | 1.29 mmHg |
| Flash point | 91 °C |
| Ignition temperature | 305 °C |
2. Chemical Reactions of Pyruvic Acid
Pyruvic acid reacts as both carboxylic acid and ketone to form derivatives such as oximes, hydrazones, and salts.
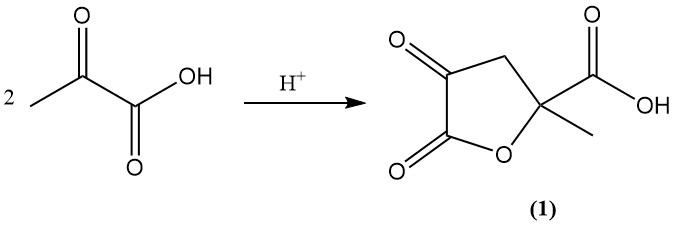
Under acidic conditions or upon standing, pyruvic acid is converted to 4,5-dioxo-2-methyltetrahydrofuran-2-carboxylic acid (1).
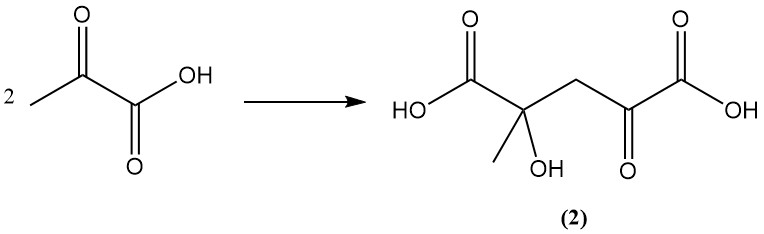
Aqueous pyruvic acid solutions undergo polymerization to higher molecular weight products via ketoglutaric acid (2) and the trimeric aldol products.
Similar to other 2-oxo acids, when treated with concentrated sulfuric acid, pyruvic acid eliminates carbon monoxide.
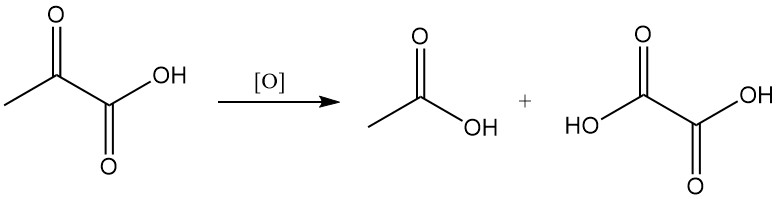
Oxidation of pyruvic acid yields acetic or oxalic acid and carbon dioxide, depending on reaction conditions.
Reduction of pyruvic acid produces lactic acid.
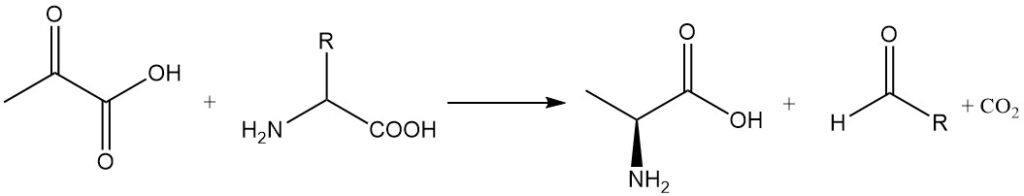
Transamination reactions between pyruvic acid and α-amino acids produce alanine and the corresponding aldehydes with one carbon atom less. Alanine can also be synthesized by reductive amination of pyruvic acid.
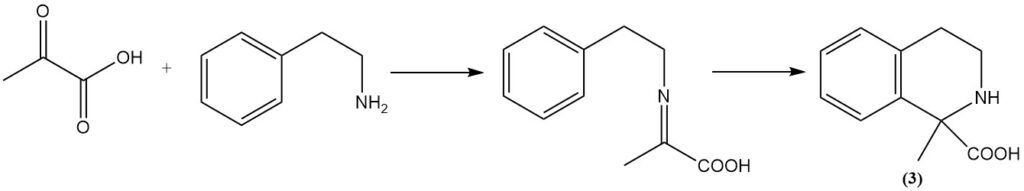
The Bischler-Napieralski reaction of phenylethylamine with pyruvic acid yields the corresponding tetrahydroisoquinoline (3).
Pyruvic acid reacts with o-phenylenediamines to form quinoxalinols and with 4,5-diaminopyrimidines to produce hydroxypteridines.
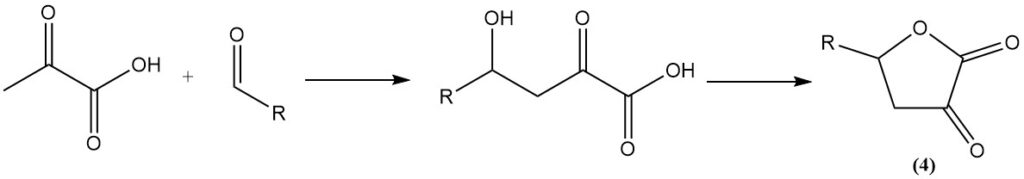
The reaction of aldehydes with pyruvic acid gives α-keto-γ-hydroxy acids, which then cyclize to butyrolactone derivatives (4).
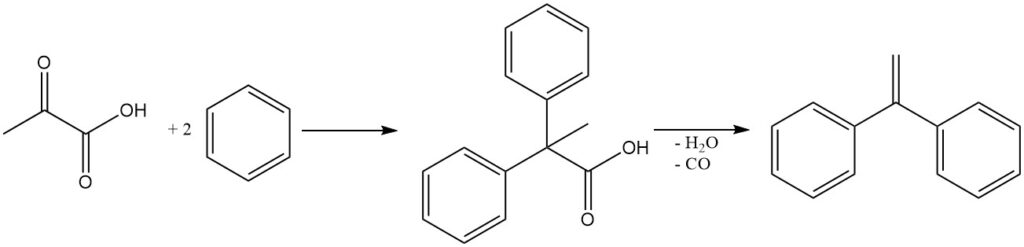
Diarylpropionic acids are produced by the Friedel-Crafts type reactions of pyruvic acid and aromatic compounds. These compounds are used as intermediates in the synthesis of 1,1-diarylethylene by dehydration and decarbonylation.
3. Industrial Production of Pyruvic acid
Industrially, pyruvic acid is produced by the dehydration and decarboxylation of tartaric acid.
In this process, pyruvic acid is distilled from a mixture of tartaric acid and potassium and sodium hydrogen sulfates at 220 °C. The crude acid obtained in approximately 60% yield undergoes subsequent vacuum distillation for purification. Ethylene glycol addition can lower the reaction temperature to 160 °C.
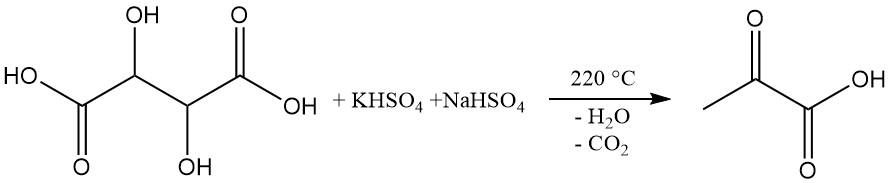
Alternative production methods include the gas-phase oxidation of lactic acid, although this process has not achieved industrial success.
In contrast, the microbial oxidation process of D-lactic acid offers a high yield. Additionally, pyruvic acid can be produced by the microbial oxidation of propylene glycol or the hydrolysis of 2,2-dihalopropionic acids. Another synthetic method is the halogen oxidation of methylglyoxal.
4. Uses of Pyruvic Acid
Pyruvic acid is used primarily as an intermediate in the synthesis of pharmaceuticals such as antimicrobial, anti-inflammatory, and cardiovascular drugs.
It is also used in the production of cosmetics for its exfoliating and skin-renewing properties. It is often used in chemical peels.
Pyruvic acid is used in the food industry as a flavoring agent. It is often employed in the production of cheese, yogurt, and other dairy-based products. It contributes to the color and flavor of bread and pastries and improves the taste of processed meats.
Beyond its flavor-enhancing properties, pyruvic acid also acts as a preservative in some food products due to its antimicrobial activity.
Other uses of pyruvic acid are in the production of crop protection agents and polymers.
5. Toxicology of Pyruvic Acid
Pyruvic acid is corrosive and irritates the eyes, skin, and respiratory system.
Acute Toxicity
- Ingestion: severe burns to the mouth and throat, with a risk of perforation of the esophagus and stomach.
- Inhalation: corrosive to the respiratory system.
- Skin contact causes severe burns.
- Eye contact causes serious eye damage.
Other Toxicological Effects
- There is no data available on dermal irritation or sensitization.
- The Ames test for mutagenicity was negative.
- IARC, NTP, and OSHA do not classify pyruvic acid as a carcinogen.
- Reproductive toxicity data is not available.
- There is no information on specific target organ toxicity or aspiration hazards.
References
- Oxocarboxylic Acids; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a18_313
- https://www.sciencedirect.com/topics/chemistry/bischler-napieralski-reaction
- https://pubchem.ncbi.nlm.nih.gov/compound/Pyruvic-Acid
- https://www.sigmaaldrich.com/US/en/sds/Aldrich/W297003
- https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/cber.19380711024
