Production of Amino Resins
1. General Process for Producing Amino Resins
Amino resin production is a two-step process: hydroxymethylation and condensation. Hydroxymethylation is the addition of formaldehyde to an amino compound, such as urea, to form a hydroxymethyl derivative. Condensation is the reaction of two hydroxymethyl derivatives to form a larger molecule.
Table of Contents
The hydroxymethylation reaction is typically carried out in a slightly alkaline or slightly acidic medium, while the condensation reaction requires a more strongly acidic solution. The exceptions are melamine–formaldehyde resins, which require an alkaline pH, and melamine–urea–formaldehyde resins, which are condensed in slightly alkaline media.
The process for the production of amino resins is continued until the resulting product is an oligomeric mixture that is still soluble or fusible. It is then protected against further condensation by rendering the medium alkaline.
The condensation of the resin by means of acid catalysis, i.e., cross-linking to give a substantially infusible product, is not carried out until the resin is put to use. This process is called curing.
Even in the cured state, free hydroxymethyl groups may be present. Monomolecular and hydroxymethyl compounds with low degrees of polymerization and up to six urea–formaldehyde units are virtually all known and can be prepared in pure form.
However, aqueous solutions of the oligomers with low degrees of polymerization do not have a sufficiently long shelf life. Only when the concentrations of hydroxymethyl and NH2 groups are reduced by partial condensation or when the products are present in solid form do they possess an industrially useful shelf life, i.e., of several weeks or more.
Condensations carried out in an excess of an alcohol, such as methanol or butanol, with the addition of acid and removal of water as necessary, produce curable etherified (i.e., alkylated) amino resins. These are soluble in non-aqueous solvents and can be mixed with alkyd resins, epoxy resins, etc. They are used as starting materials in the production of surface coatings.
Resin glues based on urea, melamine, or both have been subjected to partial etherification to stabilize them against further condensation. Historically, these resins have been used for the manufacture of plywood, but are practically not used any longer.
The basic difficulties encountered in the production of amino resins are preventing the resins from solidifying during the production process and selectively achieving certain properties, such as long shelf life, low formaldehyde content, low formaldehyde emissions from final products, strength, and resistance to swelling of particle board after curing.
1.1. Hydroxymethylation
Hydroxymethylation is the addition of formaldehyde to an amino compound, such as urea, to form a hydroxymethyl derivative. The reaction can be catalyzed by either alkaline or acidic conditions, but alkaline conditions are preferred as they result in a faster reaction rate.
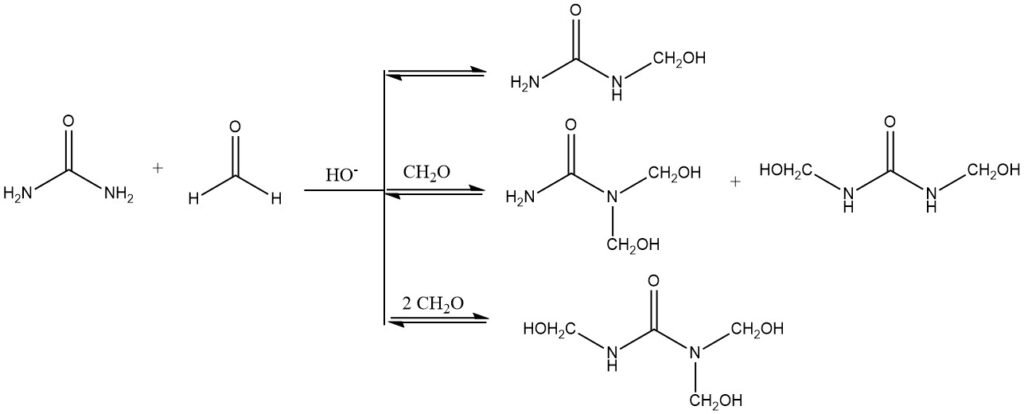
In alkaline media, the hydroxymethylation reaction proceeds through the formation of a semiaminal bond. This bond is unstable and can easily be cleaved, which means that the hydroxymethylation reaction is reversible and an equilibrium is established. This is why it is possible to isolate mono-, di-, and tri-hydroxymethylurea, but not tetrahydroxymethylurea.
The complete hydroxymethylation of all amino groups to form hexahydroxymethylmelamine is also possible under alkaline conditions. The reaction is slightly exothermic, meaning that heat is released. The enthalpy of hydroxymethylation of urea is about -23 kJ/mol.
The reaction rate of hydroxymethylation is affected by a number of factors, including concentration, temperature, pH, and the presence of substituents. In general, the reaction rate increases with increased concentration, temperature, and pH. Substituents can also have an effect, with electron-withdrawing groups and bulky substituents decreasing the reaction rate.
The reaction rates of hydroxymethylation have been measured frequently, but they are sensitive to many parameters. This makes it difficult to define an overall reaction rate for industrial processes, which produce variable mixtures of oligomers with mixed repeat units. Additionally, the important resin properties have not been correlated with the individual reaction rates.
Despite these challenges, hydroxymethylation is an important reaction in the production of amino resins. By understanding the reaction mechanism and factors affecting the reaction rate, it is possible to optimize the process and produce high-quality resins with desired properties.
1.2. Condensation
The condensation of hydroxymethyl derivatives is catalyzed by acids. Strong alkalis can also be used as catalysts, but the resulting amino resins are not important industrially. The condensation can take place through the following routes:
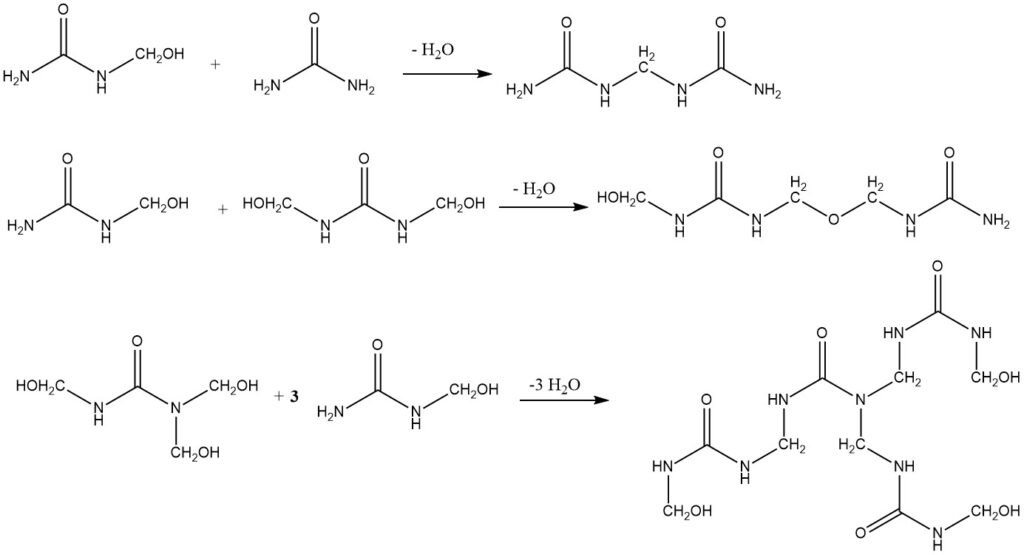
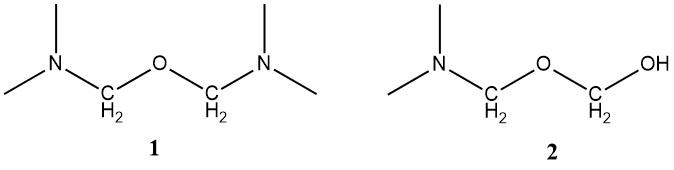
The methylene bonds between two urea molecules are very stable and can only be hydrolyzed by strong acids at elevated temperatures. The condensation reaction is slightly exothermic, with an enthalpy of -28 kJ/mol.
The stability of the methylene–ether bond is between that of the hydroxymethyl bond and that of the methylene bond. It is impossible to give a single reaction rate for the condensation process because there are many different starting materials (hydroxymethyl compounds) and many end products (methylene compounds).
The type of solvent also has an effect on the condensation rate. The dependence of the reactivity of hydroxymethyl compounds on the chemical composition of the amino compounds can be interpreted on the basis of several qualitative rules.
1.3. Composition of an Amino Resin Solution
Structural composition of oligomers in amino resin solutions can be identified by the use of 1H and 13C NMR spectroscopy. Within the spectrum, the hydrates of formaldehyde oligomers, HO(CH2O)nH, with n approximately equal to 5, are detectable.
Various hydroxymethyl structures, including ethers like hydroxymethyl methyl ether, can be distinguished by observing the parent alcohol’s corresponding C atoms. Additionally, structures (3) and (4) along with ring compounds, containing cyclic ethers of the urone type can be detected.
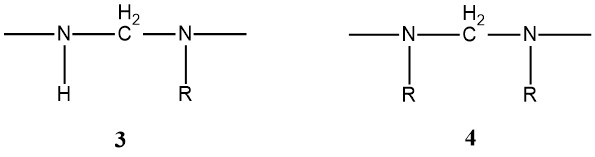
In a typical particle board glue, the 13C NMR spectrum might show, for example, the following proportions of carbon atoms from the original formaldehyde (in mole fractions):
- Formaldehyde: 0.03
- Hydroxymethyl compounds: 0.72
- Methylene compounds: 0.15
- Cyclic compounds: 0.10
Alternative analytical methods can also be applied to further elucidate the resin’s structure. For instance:
- High-Performance Liquid Chromatography (HPLC), primarily employed for low molecular mass constituents such as urea, monomethylolurea, N,N-dimethylolurea, N,N’-dimethylolurea, trimethylolurea, and methylene diurea.
- Size Exclusion Chromatography, mainly used for higher molecular mass species like dimers and oligomers.
2. Amino Resins Production Processes
Amino resins are typically produced in batches of 5-100 cubic meters. However, continuous processes are also used, especially for the production of resin glues. The patent literature describes processes in which a particular stage is operated continuously while other stages are operated batchwise.
The reaction rate and the composition of the end product are influenced by a large number of parameters, so the reaction must be carefully controlled. Important reaction parameters include:
- Purity or composition of the starting materials
- Molar ratio in each of the reaction stages
- Type and amount of modifier
- Reactant concentrations
- pH value at each stage of the reaction
- Temperature at each stage of the reaction
- Type and concentration of catalyst
- Amount of buffer salt
- Reaction time in each stage
2.1. Batchwise Production
The batchwise process is the most common method for industrially producing amino resins. It has the disadvantage of having a relatively small production capacity, but it allows for a wide variety of products and frequent changes of product.
The reactions are carried out in stirred kettles in two or more stages at 70-100 °C. In the first stage, which is carried out in a slightly acidic to alkaline solution, the main reaction is hydroxymethylation.
In subsequent stages, condensation occurs, water is eliminated, and higher molecular weight products with increasing viscosity are formed. The duration of the condensation depends on the desired properties of the product.
In general, the products are concentrated to 60-70% aqueous solutions by evaporation. The starting materials, urea or melamine, are used in powder form. Urea can also be used in solution. Formaldehyde is used as a 37-55% aqueous solution.
In a few special cases, formaldehyde is used in the solid form, paraformaldehyde. Finally, it is possible to use urea-formaldehyde precondensates in concentrations of no more than 80% by mass of the active ingredients.
2.1.1. Aqueous Solutions
A plant diagram for the batchwise production of amino resins aqueous solutions is illustrated in Figure 1. The reactor consists of a kettle equipped with heating and cooling mechanisms, a stirrer, inlets for raw material addition and measurement devices, a bottom valve for discharge, and a manhole for cleaning.
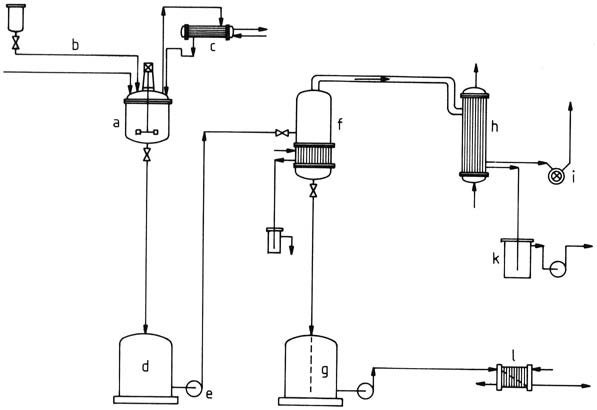
a) Stirred kettle; b) Raw material feed; c) Reflux condenser; d) Temporary container; e) Pump; f) Vaporizer; g) Container for finished product; h) Condenser; i) Vacuum pump; k) Vessel for condensed vapors; l) Cooler
The use of stainless steel, like materials St. 1.4541 or St. 1.4571, is common. Internal coils or externally affixed semicircular pipes facilitate heating and cooling. Stirring employs robust disk or anchor stirrers. Nozzles are incorporated for controlled raw material addition, temperature, and pH measurement.
Upon completion of condensation, product evaporation can take place, either in the stirred kettle or more economically in a single- or multistage tubular vaporizer. Evaporation is carried out under reduced pressure to preserve the resin.
The quality of the resin is then assessed against specifications and transferred to a storage container. The pipelines can be made of aluminum or steel, as the medium is slightly alkaline, while product tanks can be constructed from glass fiber reinforced polyester or lined iron.
For exemple, a typical procedure for producing a particle board glue involves introducing 85 parts (by mass) of solid urea into 158 parts of a neutralized 50% formaldehyde solution. The mixture is maintained at 80°C for 10 minutes to facilitate hydroxymethylation.
The pH is then adjusted to 4–5 using a 10% formic acid solution. Further condensation raises the temperature to 90–100°C within minutes. This temperature is sustained until a specific endpoint is achieved, determined either by the formation of a white precipitate or the attainment of a designated viscosity.
The reaction mixture is then alkalized slightly using a 25% sodium hydroxide solution to stop the condensation. Additional urea is added to achieve a formaldehyde-to-urea molar ratio of 1.1.
The final solution contains approximately 60% solids, which can be evaporated under reduced pressure to attain a commercial concentration of 66%.
2.1.2. Amino Resin Powders
For the production of amino resin powders, an aqueous resin solution is initially produced and then subjected to spray drying (Figure 2).
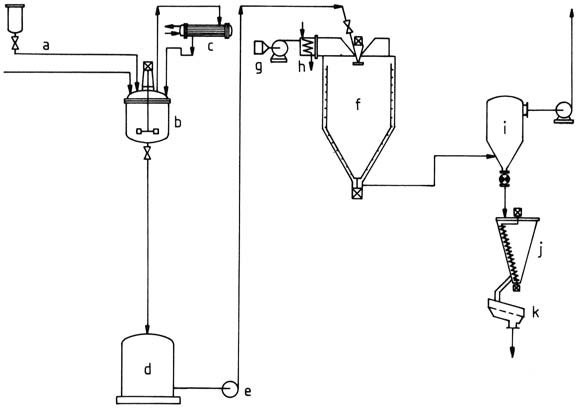
a) Starting material feed; b) Stirred kettle; c) Reflux condenser; d) Temporary container; e) Pump; f) Spray drier; g) Blower; h) Air heater; i) Filter; j) Mixing bin; k) Vibrating sieve
The solution is atomized using a spray disk or nozzle in a spray drier. Resultant droplets are dried in a stream of hot gas generated either by indirect air heating or mixing hot exhaust gas with air. The powder is collected, sieved, and then packed.
2.1.3. Etherified Products Containing Solvent
Products containing solvents, suitable for making surface coatings, can be produced in reactors similar to those used for aqueous solutions (Figure 3).
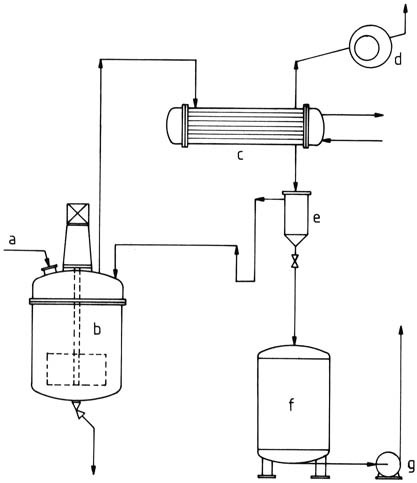
a) Raw material feed; b) Stirred kettle; c) Condenser; d) Vacuum pump; e) Separating vessel (water separator); f) Product vessel; g) Pump
A sufficiently large heat exchanger is required for vaporizing the mixture of water and excess alcohol, often butanol.
A water separator is positioned downstream from the heat exchanger. The aqueous phase that separates still contains residual formaldehyde and alcohol and needs further processing. At the appropriate alcohol content, the solvent phase is recycled to the production process.
For the production of a butylated melamine-formaldehyde resin for surface coatings, melamine and formaldehyde in a 40% aqueous solution, along with butanol, are refluxed. After distillation of water, the mixture is cooled, resin content determined, and concentration is adjusted to 50% using butanol.
2.2. Continuous Production
The continuous industrial production of amino resins was introduced to meet the growing demand for these resins. However, there are some disadvantages to continuous production.
The amount of resin produced per unit time in a particular plant can only vary within relatively narrow limits. This is because prolonged residence under constant conditions increases the proportion of molecules with high degrees of condensation.
Additionally, it is not easy to change the product being produced in a continuous process. If the plant is not cleaned out beforehand, a product with a composition between the old product and the new will be produced for a time.
For this reason, continuous production is typically used for a limited range of products. However, it does produce a very uniform product quality.
The patent literature describes a large number of continuous processes for producing amino resins. These processes differ only in the process technology for the product flow.
The differences typically involve variations in the temperature, pH, concentration, or modifiers. The process and product flow are otherwise the same.
In many cases, the publications only describe laboratory- or pilot-scale plants that have not been scaled up for industrial use.
2.2.1. Amino Resins Aqueous Solutions
The continuous production of aqueous amino resin solutions involves various apparatuses, such as tube reactors, stirred kettle cascades, or combinations of both.
Continuous processes possess a greater risk of incrustation due to highly condensed, insoluble products compared to batch processes.
In batch processes, residues are dissolved by fresh resin solutions, a mechanism that doesn’t apply as effectively in continuous processes due to limited back-mixing.
Several continuous production processes have been detailed in patent literature:
1. Girdler Corp. (1943) described a two-reactor process where reactants were mixed in the first reactor, heated under pressure, and then moved to a second stage for condensation.
2. Sherwood Paints (1949) employed a packed column for the reaction, expelling water vapor through a countercurrent gas stream.
3. Allied Chemical Corp. (1951) used a tubular coil reactor, followed by evaporation. Another process of Allied Chemical Corp. (1952) used injected steam under pressure.
4. Spumalit-Anstalt (Liechtenstein) utilized a solid catalyst (polycarbamide resin) under pressure to form the resin.
5. Rütgerswerke (Germany, 1955) employed an ion exchanger as a fixed-bed catalyst for resins used in molding compounds.
6. Skanska Attifabriken AB (Sweden, 1955) utilized a two-chamber process where starting materials underwent partial condensation in the first chamber before completing the reaction in the second chamber.
7. Du Pont (1956) described a cascading process involving two to four stirred kettles for producing etherified formaldehyde–urea resins.
8. Meissner process (1966) conducted the hydroxymethylation stage continuously while the subsequent condensation stage operated batchwise.
9. BASF (1971) depicted a process using a cascade of three or more stirred kettles for hydroxymethylation followed by condensation (Figure 4).
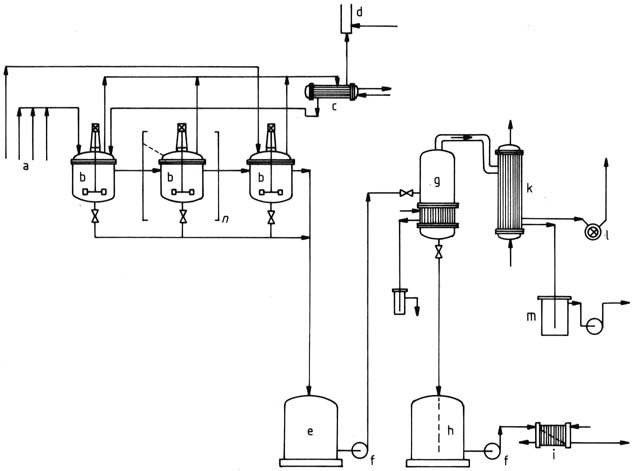
a) Starting material feeds; b) Stirred kettle; c) Reflux condenser; d) Flare; e) Temporary container; f) Pump; g) Vaporizer; h) Product container; i) Cooler; k) Condenser; l) Vacuum pump; m) Container for condensed vapors
10. Stamicarbon and American Cyanamid discussed the continuous production of concentrated, formaldehyde-rich melamine–formaldehyde solutions for amino resin starting materials.
11. DSM outlined a continuous process where condensation resin is prepared in an aqueous medium at elevated temperatures and pressure, utilizing a tubular reactor with static mixer elements.
Each of these processes demonstrates the diverse approaches taken to achieve continuous amino resin production while managing challenges like incrustation and product variety.
2.2.2. Resins in Powdered Form
Powdered resins can also be produced continuously by feeding the spray solutions, which are typically produced in batches, continuously from a temporary container into a spray tower.
The spray tower is a tall, narrow chamber where the spray solutions are atomized into fine droplets. The droplets are then dried by hot air or gas. The dry resin powder is collected at the bottom of the tower.
This process is continuous because the spray solutions are fed into the tower continuously. This allows for a more consistent product quality than the batch process.
2.3. Production of Special Products and Foams
In addition to impregnating resins, other special products include foamed resins and resins for paper auxiliaries, label adhesives, concrete liquefiers, leather auxiliaries, carpet coatings, microcapsules, and so on. These resins are typically produced in batches because they are produced in relatively small quantities and there is a wide variety of products.
The production of foamed resins is similar to the production of resin glues. The proprietary know-how for these products relates to modifiers and small but important variations in the process.
Foaming resins are an interesting type of amino resin that is used to produce solid foams. These foams have a wide variety of applications, including heat insulation, sound insulation, soil conditioning in agriculture, filling of mining cavities, carrier material for cleaning fluids, and covering for landfills.
The first foamed resins were produced in the 1930s. The process involves adding a foaming agent and air to a glue solution. The foaming agent causes the solution to form bubbles, and the air helps to expand the bubbles.
The resin then cures, forming a solid foam. The dried foamed urea formaldehyde resins had bulk densities ranging from 5 to 70 kg/m3, with more than 60% of the foam adopting the form of open cells.
The development of portable apparatus for on-site production of foamed resins led to much wider applications for these products. Today, foamed resins are used in a variety of industries, including construction, automotive, and packaging.
References
- Amino Resins; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_115.pub2
- Amino resin and a method for its production. – https://patents.google.com/patent/EP0277106B1/en
- Amino resins; Encyclopedia of Polymer Science and Technology. – https://onlinelibrary.wiley.com/doi/abs/10.1002/0471440264.pst017
