o-Cresol: Properties, Reactions, Production and Uses

What is o-Cresol?
o-Cresol, also known as 2-methylphenol, is an organic compound with the chemical formula C7H8O. It is a colorless crystalline solid at room temperature with a phenolic odor and is highly soluble in most organic solvents.
Table of Contents
1. Physical Properties of o-Cresol
Pure o-cresol is a colorless crystalline solid at ambient temperature; however, it becomes yellow to brown after storage for a period of time. O-cresol has a phenolic odor and absorbs moisture from the air.
O-cresol is highly soluble in phenol and in many organic solvents such as aliphatic alcohols, ethers, chloroform, and glycerol and is less soluble in water than phenol.
O-cresol can be distilled with steam and forms azeotropes with a number of compounds, e.g., decane, 1-decene, 1-undecene, dodecane, 1,2,4,5-tetramethylbenzene, divinylbenzene, ethylene glycol, diethylene glycol methyl, and ethyl ether.
Important physical properties of o-cresol are summarized in the following table:
| Property | Value |
|---|---|
| CAS number | [95-48-7] |
| Chemical formula | C7H8O |
| Molecular weight | 108.14 g/mol |
| Melting point at 101.3 kPa | 30.99 °C |
| Boiling point at 101.3 kPa | 191.00 °C |
| Density at 25 °C | 1.135 g/cm3 |
| Density at 50 °C | 1.0222 g/cm3 |
| Viscosity at 50 °C | 3.06 mPa·s |
| Refractive index at 50 °C | 1.5310 |
| Dielectric constant at 50 °C | 6.00 |
| Specific conductance at 50 °C | 0.43×10-10 S/cm |
| Surface tension at 50 °C | 34.4×10-6 N/m |
| Dissociation constant at 25 °C | 4.8×10-11 |
| Critical temperature | 424.4 °C |
| Critical pressure | 5.01 MPa |
| Critical density | 384 kg/m3 |
| Heat capacity cp at 25 °C (gas) | 127.3 J mol-1 K-1 |
| Heat capacity cp at 25 °C (solid) | 154.7 J mol-1 K-1 |
| Heat capacity cp at 50 °C (liquid) | 237.9 J mol-1 K-1 |
| Enthalpy of fusion ΔHm at melting point, 101.3 kPa | 15.830 kJ/mol |
| Enthalpy of sublimation ΔHsub at 25 °C, 101.3 kPa | 76.07 kJ/mol |
| Enthalpy of vaporization ΔHv at boiling point, 101.3 kPa | 45.222 kJ/mol |
| Enthalpy of combustion ΔH°c at 25 °C, 101.3 kPa | - 3696 kJ/mol |
| Enthalpy of formation ΔH°f at 25 °C, 101.3 kPa | -204.8 kJ/mol |
| ΔH°f at 25 °C (gas), 101.3 kPa | - 128.7 kJ/mol |
| Free energy of formation ΔG°f at 25 °C, 101.3 kPa | - 55.7 kJ/mol |
| ΔG°f at 25 °C (gas) and 101.3 kPa | - 33.0 kJ/mol |
| Entropy S at 25 °C and 101.3 kPa | 165.5 J mol-1 K-1 |
| Entropy S° at 25 °C (gas) and 101.3 kPa | 352.8 J mol-1 K-1 |
| Solubility in water at 25 °C | 2.6 wt % |
| Solubility in water at 100 °C | 4.8 wt % |
| Distribution coefficient log P octanol/water at 25 °C | 1.95 |
| Vapor density (air = 1) | 3.72 |
| Flash point (closed cup) | 81 °C |
| Ignition temperature | 555 °C |
| Flammability limit, lower, in air at 20 °C, 101.3 kPa | 1.3 vol% |
2. Chemical Reactions of o-Cresol
o-Cresol is chemically similar to phenol. It is weak acid and dissolves in aqueous alkali to form water-stable salts known as o-cresolates. Therefore, they can be extracted into a sodium hydroxide solution from solvents that are not miscible with water.
The hydroxyl group of o-cresol can be etherified with alkyl halides, dialkyl sulfates, dialkyl carbonates, and toluenesulfonic acid esters and react with acyl anhydrides or acyl chlorides to form the corresponding tolyl esters, which are normally referred to as ‘‘o-cresyl’’ esters.
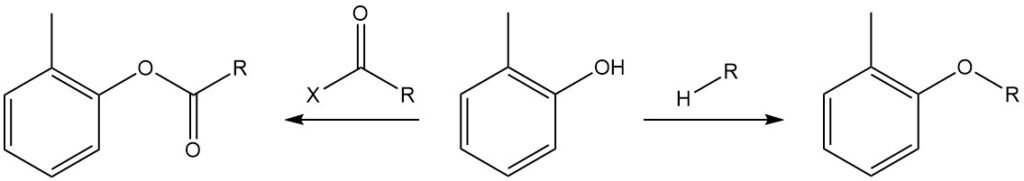
The OH group of o-cresol reacts with isocyanates to form urethanes.
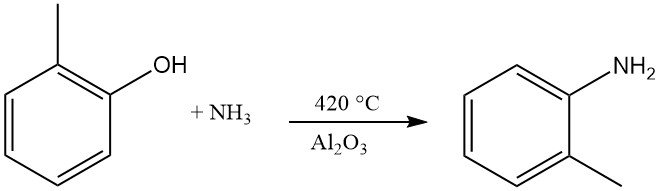
o-Toluidine is formed by the reaction of o-cresol with ammonia at a temperature of 420 °C and in the presence of Al2O3.
Different 2-halogenotoluenes can be obtained by replacing the hydroxyl group of o-cresol. Examples include the reaction with sulfur oxytetrafluoride at 150 °C, with diphenylphosphine trichloride at 220 °C, or with phosphorus tribromide at 280 °C to produce the corresponding 2-fluoro-, 2-chloro-, or 2-bromotoluene, respectively.
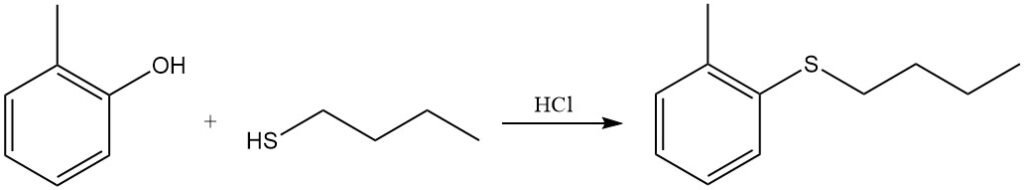
The phenolic OH group of o-cresol can be replaced by a thiobutyl group by reaction with butanethiol in hydrochloric acid.
Toluene is produced by distillation of cresol with zinc powder and can also be formed by hydrogenation in the vapor phase at 300-400 °C under pressure (up to 8 MPa) in the presence of catalysts (transition metals and aluminum oxide).
Hydrodealkylation of o-cresol to phenol can be achieved using catalysts at 400–500 °C or purely thermally at 500–700 °C.
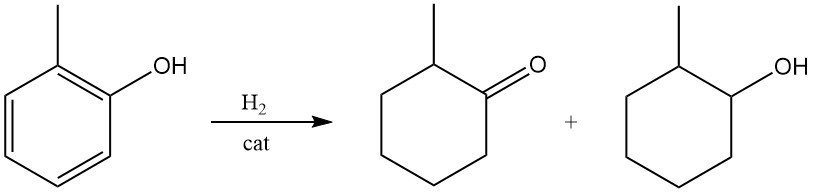
2-Methylcyclohexanone or 2-methylcyclohexanol can be formed under mild conditions by hydrogenation of o-cresol over Raney nickel or noble metal catalysts.
o-Cresol is sensitive to oxidation and, depending on the oxidizing agent and reaction conditions, yields a large number of compounds such as hydroquinones, quinols, quinones, cyclic ketones, furans, dimeric and trimeric cresols, and tolyl ethers.
If the hydroxyl group of o-cresol is protected by esterification or etherification, the methyl group can be selectively mono-, di-, or trichlorinated, or selectively oxidized to a formyl group (e.g., with manganese dioxide or oxygen) or to a carboxyl group (salicylic acid) with acid permanganate solution.
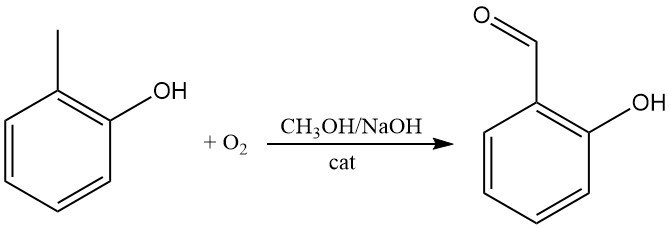
o-Cresol can be directly oxidized with oxygen to give 2-hydroxybenzaldehyde, e.g., in methanolic sodium hydroxide in the presence of catalytic amounts of iron tetraarylporphines.
Alkali fusion of the o-cresol in the presence of lead oxide or manganese dioxide produces 2-hydroxybenzoic acid directly.
Similar to phenol, o-cresol readily undergoes electrophilic substitution mainly in the o- and p-positions relative to the hydroxyl group. Therefore, it can be nitrated even with dilute nitric acid.
Nitrosation, halogenation, sulfonation, alkylation, and acylation occur readily. When o-cresol is heated with Friedel-Crafts catalysts, such as AlCl3, it isomerizes to m-cresol, thermodynamically the most stable isomer.
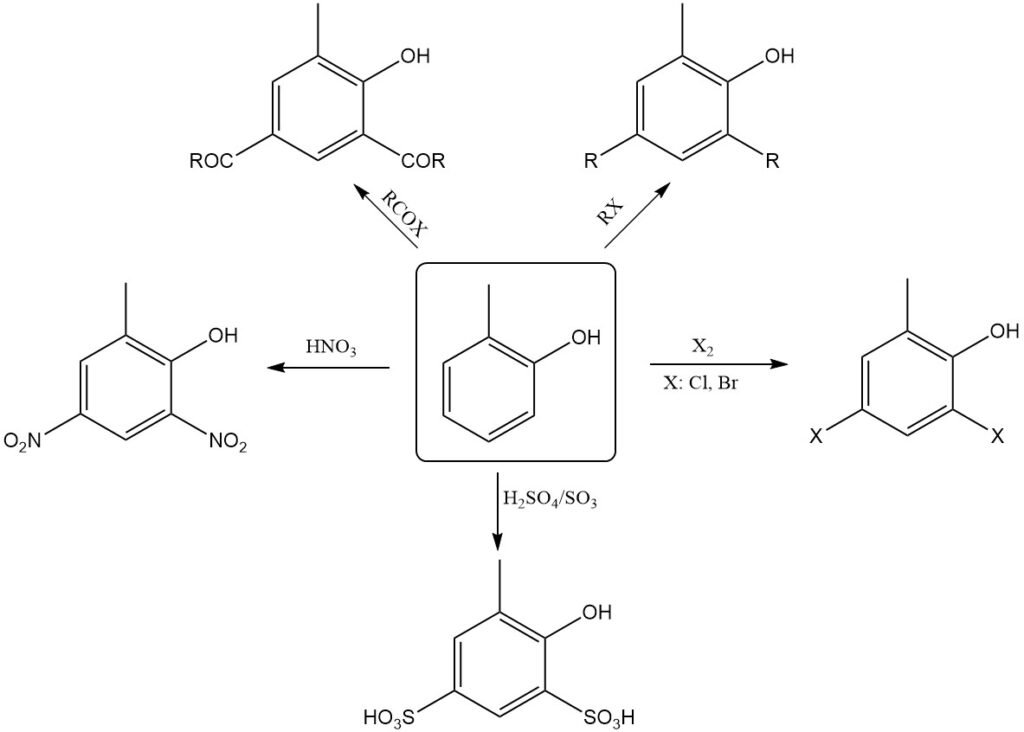
The heating of dry alkali metal o-cresolates with CO2 under pressure produces hydroxy(methyl)benzoic acids (Kolbe-Schmitt reaction).
The reaction of formaldehyde with o-cresol in the presence of alkali at room temperature produces hydroxy(methyl)benzyl alcohols, which under acidic conditions or at elevated temperatures condense to form high-molecular resins.
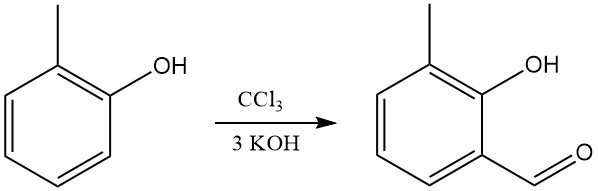
o-Cresol reacts with chloroform and alkali to form mainly o-hydroxy(methyl)benzaldehydes (Reimer-Tiemann synthesis).
o-Cresol shows aggressive corrosive properties towards various metals, particularly at elevated temperatures. The extent of corrosion is influenced by the presence of water in the cresol solution.
Chromium-nickel stainless steels and chromium steels offer limited resistance to corrosion by o-cresol. Nickel exhibits superior corrosion resistance, while tantalum is considered entirely resistant.
Aluminum and its alloys are susceptible to severe corrosion, especially at low water content, and copper and brass alloys should be avoided due to their susceptibility to corrosion.
3. Formation and Isolation of Cresol
Cresols (a mixture of ortho, meta, and para isomeres) were historically obtained exclusively from coal tar. Following World War II, spent refinery caustics emerged as an additional source. Since the mid-1960s, synthetic production of cresols has been significantly growing.
Currently, “synthetic cresol” accounts for approximately 60% of the demand in the United States, Europe, and Japan, and “natural cresol,” derived from coal tar and spent refinery caustics, is only about 40%.
A mixture of cresols, xylenols, and a variety of other phenolic compounds are found in the wastewater generated from cracking processes, tars, and tar-like products derived from thermal cracking, oxidizing thermal cracking, and hydrogenating thermal cracking of natural materials such as bituminous coal, lignite, oil shale, peat, wood, lignin, and other biomaterials.
The yields and relative quantities of cresols, xylenols, and other phenols produced depend not only on the starting material but also on the process conditions, including temperature, residence time, reactor type, and operational mode.
For bituminous coal, hydrogenation yields the highest amounts, followed by low-temperature carbonization, while high-temperature coking results in the lowest yields. Gasification of lignite produces significantly more phenols, cresols, and xylenols compared to bituminous coal.
Small quantities of cresols, xylenols, and other phenol derivatives are also formed during the catalytic and thermal cracking of petroleum fractions, particularly coking thermal cracking.
Currently, cresols and xylenols are primarily isolated from coal tars obtained through high-temperature coking, low-temperature carbonization, Lurgi pressure gasification of coal, and spent refinery caustics.
3.1. Isolation of cresol from Coal Tars
Historically, coal tar has been a significant source of cresols and xylenols. However, its production has been steadily declining over the past four decades, with an estimated annual output of 30,000 to 40,000 tons.
High-temperature coke-oven tar, a byproduct of metallurgical coke production, contains approximately 0.4-0.6% phenol, 0.8-1% cresols, and 0.2-0.5% xylenols.
In the United Kingdom, cresols were also produced from low-temperature coal tars obtained in the production of smokeless fuels. However, the availability of these tars significantly decreased by the 1990s.
Currently, the largest source of “natural” cresols is the liquid byproducts obtained from the gasification of bituminous coal using the Lurgi process. This process is primarily used in South Africa for the production of synthesis gas for Fischer-Tropsch plants.
The liquid byproducts from this process, which are similar in composition to low-temperature tars, yield an estimated 35,000 to 55,000 tons of cresols and xylenols annually.
In the United States, the liquid coproducts from the Lurgi gasifiers of the Dakota Gasification Company, which gasifies lignite to produce synthetic natural gas, are a significant source of “natural” cresols. The estimated annual production from this source is approximately 5,000 tons of o-cresol and 11,000 tons of m/p-cresol.
To isolate cresols and xylenols from coal tar, extraction with sodium hydroxide solution is commonly employed. In the Lurgi phenoraffin process, carbolic oil, which boils at 180–210 °C, and the filtrate of naphthalene oil are extracted to obtain the phenols.
The hydrocarbons and pyridine bases present in the crude phenolate caustic are removed by steam distillation, followed by the liberation of crude phenol with carbon dioxide.
Phenolate caustics from coking plant effluents, which primarily contain phenol and cresol with minimal amounts of xylenols, are often incorporated into the extraction process. This can result in a wide variation in the composition of the resulting crude phenol.
For example, crude phenol obtained from German high-temperature coke-oven coal tar may contain 15% water, 30% phenol, 12% o-cresol, 18% m-cresol, 12% p-cresol, 8% xylenols, and 5% trimethylphenols along with higher boiling phenols.
To further purify the cresylic acid fractions from neutral oils, tar bases, sulfur compounds, and undesirable phenolic substances, an extractive distillation with diethylene glycol has been developed by the Dakota Gasification Co.
Companies such as Sasol Phenolics, Merichem Co., Coalite Chemicals, Rütgers-VfT AG, DEZA Corporation, and several Japanese companies use this process to isolate cresols and xylenols from coal tar.
3.2. Recovery of Cresol from Spent Refinery Caustics
In the United States, cresols and xylenols are obtained from naphtha fractions produced during catalytic and thermal cracking in the petroleum industry. These fractions contain approximately 0.1% of C6-C8 phenols. During the scrubbing process to remove sulfur compounds from these fractions, cresols and xylenols are also extracted using concentrated alkaline solutions.
The spent cresylate caustics obtained from this process vary widely in composition, typically containing 20-25% C6-C8 phenols and 10-15% sulfur compounds.
Until the early 1990s, these caustics were collected and reprocessed by specialized companies. However, due to increased competition from synthetic o-cresol producers, stricter environmental regulations, and a shortage of raw materials, many of these companies have closed their operations.
Merichem is currently the only major processor of spent refinery caustics in the United States.
At Northwest Petrochemical, a former processor, thiols in the alkaline solution were oxidized with air to produce disulfides, which were then decanted. The remaining phenols were precipitated using carbon dioxide and decanted. Any remaining phenols were extracted with an organic solvent and then re-extracted with an aqueous alkaline phase.
This process yielded a phenol mixture containing approximately 20% phenol, 18% o-cresol, 22% m-cresol, 9% p-cresol, 28% xylenol, and 3% higher phenols. This mixture was then separated through distillation.
The Merichem Company uses hydrogen sulfide, a waste product from refineries, to precipitate phenols instead of carbon dioxide. The sulfide solution obtained is concentrated to produce sodium sulfide for use in the paper industry and ore dressing.
The water vapor condensate formed during this process is stripped with natural gas to remove odorous compounds and then passed through a cooling tower. The natural gas is used as fuel in the final process air incinerator.
The plant’s water is recycled in an integrated system, with most of the water released into the environment as water vapor from cooling towers.
The Pittsburgh Consolidated Chemical Company previously used a process involving direct treatment of spent refinery caustics with flue gas containing carbon dioxide. The precipitated crude phenol was then distilled and extracted with aqueous methanol-light naphtha.
After removing thiol and base residues with an ion-exchange resin, the cresylic acids were recovered through fractional distillation.
Merichem‘s cresylics capacity was approximately 55,000 tons per year in 1996. However, this figure includes phenol, xylenols, and other alkylphenols. Merichem has expanded its operations to include processing cresylics from various sources, including coal gasification plants.
The recovery of cresols and xylenols from spent refinery caustics has primarily been confined to the United States. In Germany, different refinery techniques and the production of cresol-containing spent caustics may not be suitable for economical reprocessing. Additionally, the shift towards hydrotreating and the Merox process in refineries has reduced the production of spent caustics and the recovery of cresols.
4. Industrial Production of o-Cresol
Ortho-cresol (o-cresol) is primarily produced through synthetic methods, as traditional recovery from coal tar and refinery caustics is insufficient to meet global demand. These processes yield a mixture of cresol isomers. From these mixtures, only o-cresol can be separated as a pure product by distillation.
Several industrial processes are employed, often starting from toluene or phenol:
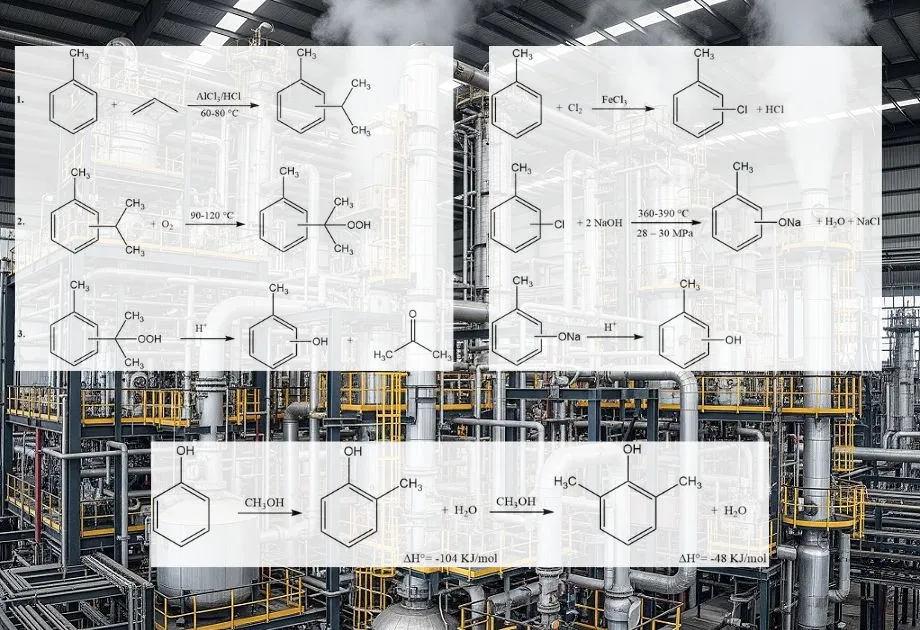
- Vapor-Phase Methylation of Phenol: This is a widely favored industrial method. Phenol reacts with methanol in the gas phase over a solid acid catalyst, such as alumina, magnesium oxide, or gallium oxide. The reaction typically occurs at elevated temperatures (e.g., 200-400 °C). This process selectively yields o-cresol and 2,6-xylenol.
- Alkaline Chlorotoluene Hydrolysis: This method involves the hydrolysis of 2-chlorotoluene in an alkaline medium. The chlorine atom is replaced by a hydroxyl group, leading to the formation of o-cresol. This process can produce a mixture of cresol isomers, with varying ratios depending on the specific conditions and catalysts used.
- Alkali Fusion of Toluenesulfonates: This process uses toluene as raw material, which is first sulfonated to form toluenesulfonic acid. The sodium salt of toluenesulfonic acid then undergoes alkali fusion (reaction with molten sodium hydroxide) to produce sodium cresoxide, which is subsequently acidified to yield cresol.
- Splitting of Cymene Hydroperoxide: This process, also starting from toluene, involves the formation of cymene (isopropyltoluene), which is then oxidized to cymene hydroperoxide. The subsequent acid-catalyzed decomposition (splitting) of cymene hydroperoxide yields cresol and acetone.
For a comprehensive and detailed article on cresol production, refer to the following article.
5. Uses of o-Cresol
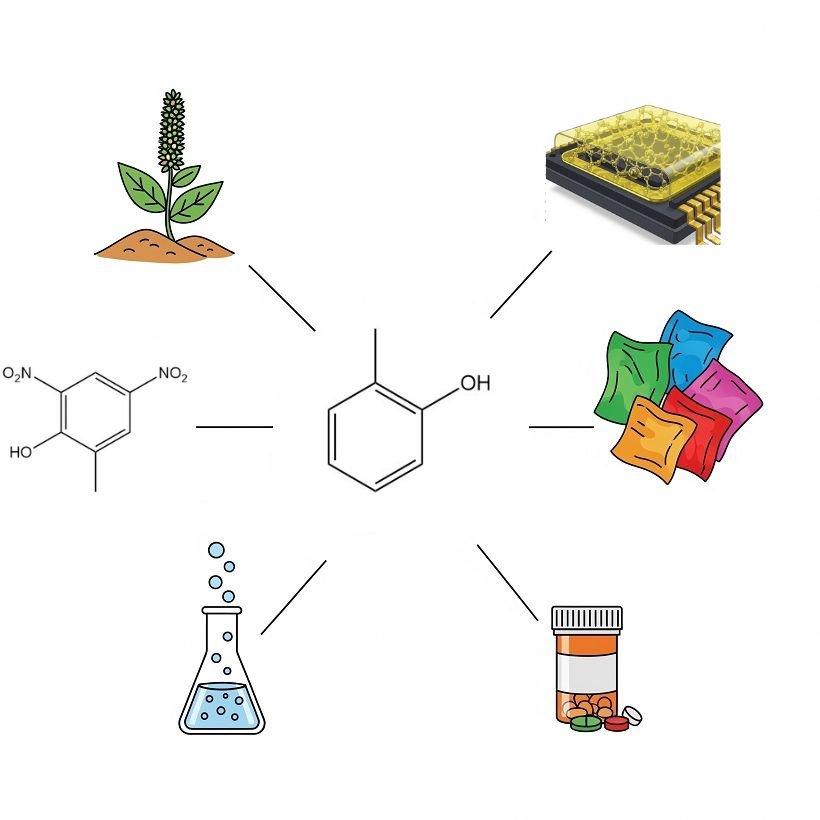
o-Cresol serves as a versatile chemical intermediate and solvent across various industries.
A primary application in Europe involves its chlorination to 4-chloro-o-cresol, which is used as a precursor for chlorophenoxyalkanoic acids, including 4-chloro-2-methylphenoxyacetic acid, 2-(4-chloro-2-methylphenoxy)-propionic acid, and γ-(4-chloro-2-methylphenoxy)butyric acid, all known as selective herbicides.
A smaller quantity of o-cresol undergoes nitration to yield 4,6-dinitro-o-cresol. This derivative exhibits both herbicidal and insecticidal properties. It is also used as a polymerization inhibitor during the production and distillation of styrene.
High-purity o-cresol is increasingly utilized, particularly in Japan, in the manufacturing of epoxy-o-cresol novolak resins (ECN resins). These resins are employed as sealing materials for integrated circuits. o-Cresol of standard quality is used in the modification of conventional phenol-formaldehyde resins.
o-Cresol is also an important precursor for various dye intermediates. Quantitatively, o-cresotinic acid (o-hydroxymethylbenzoic acid), synthesized via the Kolbe reaction, is the most significant. This acid is subsequently used in pharmaceutical production, while its methyl esters are used as dyeing auxiliaries.
A considerable amount of o-cresol is utilized as a solvent, either directly or following hydrogenation to 2-methylcyclohexanol or 2-methylcyclohexanone. Its carbonate ester form serves as a starting material in coumarin synthesis.
Alkylation of o-cresol with propene produces carvacrol (3-isopropyl-6-methylphenol), which is employed as an antiseptic and in fragrances.
Minor amounts of o-cresol are alkylated with isobutene, serving as starting materials for diverse antioxidants, components for thermal recording materials, and pharmaceuticals, such as the muscle relaxant Mephenesin.
References
- Cresols and Xylenols; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a08_025
- Cresols; Encyclopedia of Toxicology, Volume 1. – https://www.sciencedirect.com/science/article/abs/pii/B9780123864543002967
