Methylamine: Properties, Reactions, Production and Uses
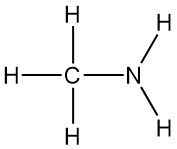
Methylamine is the simplest primary amine, with the formula CH3NH2. It’s a colorless gas that’s derived from ammonia, with one hydrogen atom replaced by a methyl group. It was first synthesized in 1849 by Wurtz alongside dimethylamine and trimethylamine.
The industrial synthesis of methylamines from methanol and ammonia was reported in 1884, and it was commercialized in the 1920s for leather tanning.
Table of Contents
1. Physical Properties of Methylamine
Methylamine is a gas that has a characteristic fishy odor at low concentrations but, at higher concentrations, exhibits an ammonia-like odor. Its aqueous solutions form crystalline hydrates upon cooling with the formula CH3NH2·3 H2O.
Methylamine is soluble in various organic solvents, such as methanol, ethanol, dimethylformamide, and ethylene glycol.
Table 1 presents the detailed physical properties of methylamine.
| Property | Value |
|---|---|
| Molecular weight (g/mol) | 31.06 |
| Boiling point (101.33 kPa), °C | -6.3 |
| Melting point, °C | -93.0 |
| Density (gas), g/cm3 at 101.33 kPa | 0.0014 |
| Density (liquid), g/cm3 at 25 °C | 0.6624 |
| pKa (at 25 °C) | 10.62 |
| Surface tension (25 °C), 10-3 N/m | 19.19 |
| Heat of vaporization (25 °C), kJ/mol | 24.249 |
| Heat of vaporization (boiling point), kJ/mol | 26.0 |
| Heat of fusion, kJ/mol | 6.054 |
| Standard heat of formation (liquid, 25 °C), kJ/mol | -47.31 |
| Standard heat of formation (gas, 25 °C), kJ/mol | -22.98 |
| Heat of combustion (liquid, standard state, 25 °C), kJ/mol | -1061.35 |
| Heat capacity (ideal gas, 25 °C), J K-1 mol-1 | 50.1 |
| Critical temperature, °C | 156.9 |
| Critical pressure, MPa | 7.46 |
| Dielectric constant (liquid, 25 °C) | 9.4 |
| Dipole moment in benzene (25 °C), D | 1.47 |
| Flash point (closed cup), °C | -62 |
| Ignition temperature in air, °C | 430 |
| Lower explosion limit in air (vol %) | 4.9 |
| Upper explosion limit in air (vol %) | 20.7 |
2. Chemical Reactions of Methylamine
Methylamine is a weak base that readily forms salts with acids (e.g., methylammonium halides, also known as methylamine hydrohalides, with the corresponding hydrohalic acids).
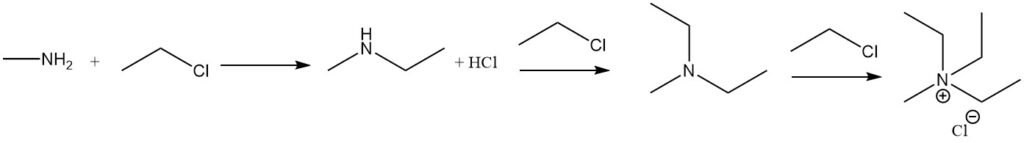
The reaction of methylamine with alkyl halides leads to further alkylation, up to the formation of quaternary ammonium salts. An example is the reaction with ethyl halide to form methylethylamine, methyldiethylamine, and methyltriethylammonium halide.
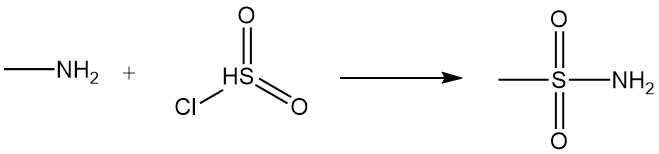
The reaction of methylamine with carboxylic acids and their derivatives results in N-methylamides, and the reaction with sulfonyl chloride produces substituted methylsulfonamide.
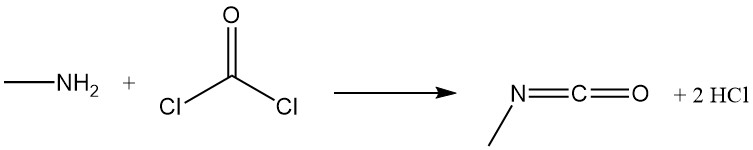
Methylamine reacts with phosgene to form methyl isocyanate, an intermediate in the production of carbamate insecticides, and with carbon disulfide in the presence of sodium hydroxide to produce sodium methyldithiocarbamate.
Urea reacts with methylamine via intermediate ammonium cyanate to yield N-methylurea.
Alkaline hypochlorites Lead to a mixture of methyl mono- and dichloroamines.
Methylamine is decomposed to methanol and nitrogen by treatment with nitrous acid and to methyl chloride and nitrogen by the action of nitrosyl chloride.
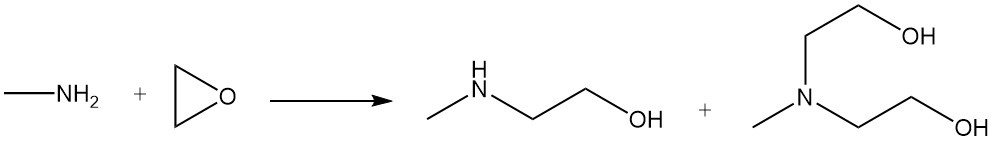
Methylethanolamine and methyldiethanolamine are produced by the reaction of methylamine with ethylene oxide.
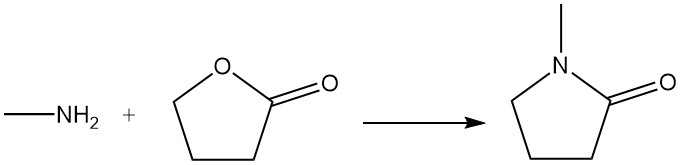
γ-Butyrolactone reacts with methylamine to produce N-methylpyrrolidone, which is used as a solvent.
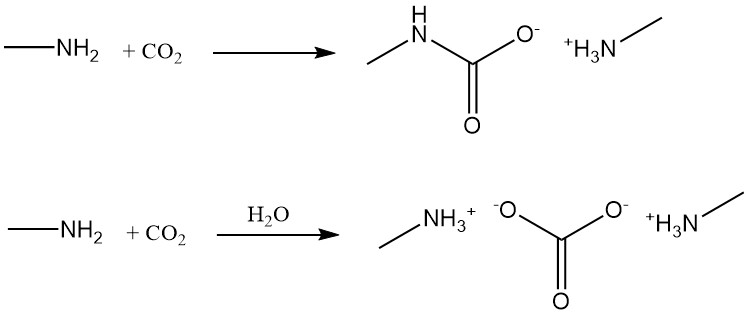
Methylamine reacts with carbon dioxide to form methylamine carbamate under anhydrous conditions and methylamine carbonate in the presence of water.
Methylamine is corrosive to aluminum, copper, copper alloys, galvanized metal, magnesium, zinc, and their alloys. However, steel demonstrates good performance as a construction material for handling both anhydrous methylamine and its aqueous solutions.
3. Production of Methylamine
Methylamine is produced via the exothermic reaction between methanol and ammonia over an amorphous silica-alumina catalyst at 350–450 °C. This reaction generates a mixture of the three methylamine variants (mono-, di-, and trimethylamine).
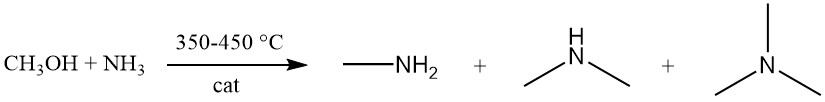
While equilibrium favors trimethylamine formation, market demand prioritizes mono- and especially dimethylamine. The amine synthesis process can be modeled by nine bimolecular equilibrium reactions, with three specifically involving amine formation:
- NH3 + CH3OH → CH3NH2 + H2O
- CH3NH2 + CH3OH → (CH3)2NH + H2O
- (CH3)2NH + CH3OH → (CH3)3N + H2O
Two primary catalyst systems are currently used for the vapor-phase methanol-ammonia reaction: amorphous solid acid catalysts and shape-selective zeolite-based catalysts.
The crude reaction mixture containing methylamines, excess ammonia, water, and unconverted methanol is purified using a series of distillation columns (typically 4-5). Trimethylamine is separated from the mixture using extractive distillation.
This purification process achieves high yields (>98%) of pure, anhydrous mono-, di-, and trimethylamine. The recovered methanol improves process efficiency and catalyst lifespan.
4. Uses of Methylamine
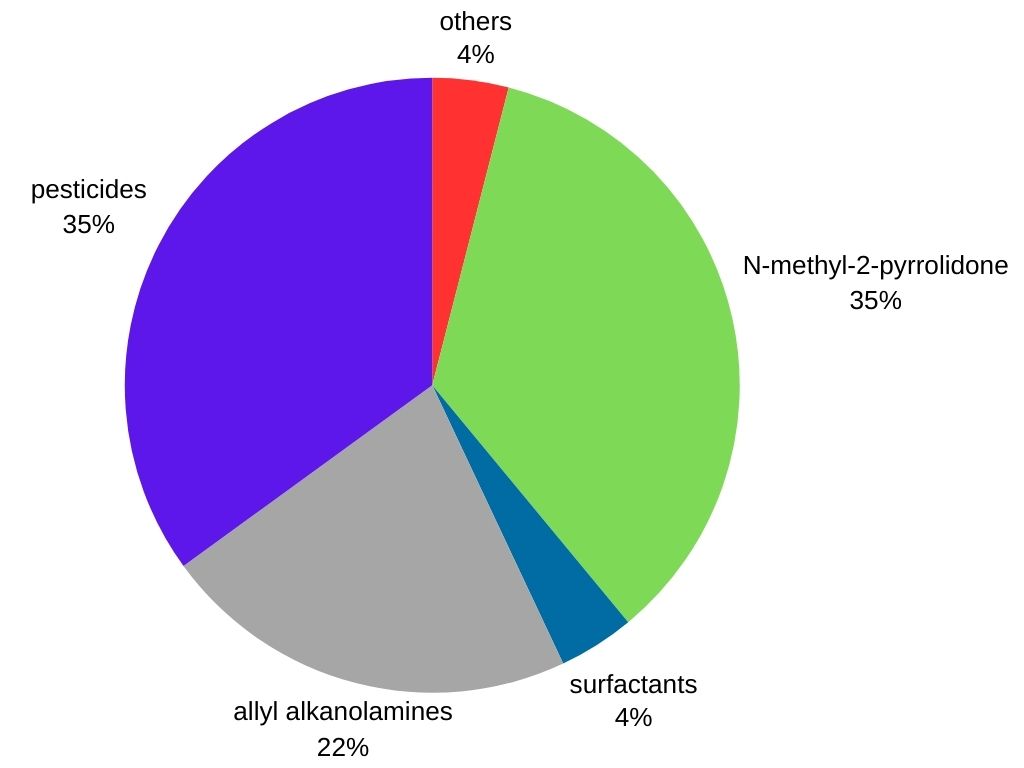
Methylamine is mainly used as an intermediate in the production of several commercial products:
Agrochemicals: Methylamine is an important intermediate in the production of numerous herbicides, pesticides, and insecticides, contributing to crop protection.
Pharmaceuticals: It is used in the synthesis of certain medications, including decongestants and antihistamines.
Rubber Processing Chemicals: Methylamine finds application in the manufacturing of various rubber products like tires and hoses.
Surfactants: It is employed in the production of cleaning and wetting agents present in detergents, shampoos, and emulsifiers.
Other Applications: Methylamine is also a precursor for Tovex (water gel explosive), the solvent N-methyl-2-pyrrolidone, methyldiethanolamine (a solvent used in hydrocarbon processing), and the soil disinfectant “Metam sodium”.
5. Toxicology of Methylamine
Both methylamine and its solutions are extremely flammable and require proper handling to prevent fire and explosion.
Methylamine and its aqueous solutions are corrosive and can cause burns and irritation to the eyes, skin, and respiratory system. When inhaled, it affects the upper respiratory tract at low concentrations (75 ppm) and higher concentrations can damage the liver.
Exposure to methylamine can cause effects similar to those of ammonia, including bronchitis, conjunctivitis, dermatitis, and, in severe cases, irritation, burns, lung damage, and delayed organ scarring.
Repeated exposure to methylamine in animals has been linked to liver toxicity, blood chemistry abnormalities, and lung issues.
Studies on genetic toxicity are not conclusive.
Existing studies suggest that the fetus is not particularly susceptible to exposure, although more research is needed.
The American Conference of Governmental Industrial Hygienists (ACGIH) has set a threshold limit value (TLV) of 5 ppm (parts per million) as a time-weighted average (TWA) and 15 ppm as a short-term exposure limit (STEL) for methylamine.
References
- Methylamines; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a16_535.pub4
- Methylamine; Encyclopedia of Toxicology (Third Edition). – https://www.sciencedirect.com/science/article/abs/pii/B9780123864543005182
- Methylamines; Kirk-Othmer Encyclopedia of Chemical Technology. – https://onlinelibrary.wiley.com/doi/10.1002/0471238961.1305200820211818.a01.pub2
