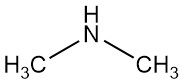
Dimethylamine is an organic compound with the formula (CH3)2NH. It is a colorless, flammable gas with an ammonia-like odor, although at low concentrations it can smell like fish, and its odor is more potent than methylamine and less than trimethylamine. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%.
Table of Contents
1. Physical Properties of Dimethylamine
Dimethylamine is a harmful, colorless gas or compressed liquid that is highly flammable, and has very strong fishy or ammonia-like odors. It is water-soluble and is sold as either aqueous solutions or in pure form.
On cooling aqueous solutions of dimethylamine, the crystalline hydrate (CH3)2NH·7 H2O is formed. Dimethylamine is soluble in various organic solvents.
Aqueous solutions of dimethylamine are good solvents for many inorganic and organic compounds; however, the potential reactivity of the solute with dimethylamine must be considered.
Table 1 lists the physical properties of dimethylamine.
| Property | Value |
|---|---|
| Molecular weight, g/mol | 45.08 |
| Boiling point (101.33 kPa), °C | 6.8 |
| Melting point, °C | -92.2 |
| Densityat 25 °C (liquid), g/cm3 | 0.6556 |
| pKa (at 25 °C) | 10.77 |
| Refractive index at 17 °C | 1.350 |
| Surface tension (25 °C), 10-3 N/m | 16.33 |
| Heat of vaporization at 25°C, kJ/mol | 23.663 |
| Heat of fusion, kJ/mol | 5.945 |
| Standard heat of formation, kJ/mol at 25 °C (liquid) | -43.96 |
| Standard heat of formation, kJ/mol at 25 °C (gas) | -18.46 |
| Heat of combustion, standard state at 25°C, liquid, kJ/mol | -1744.63 |
| Heat capacity, ideal gas (25°C), J K-1 mol-1 | 70.7 |
| Critical temperature, °C | 164.5 |
| Critical pressure, MPa | 5.31 |
| Dielectric constant (25°C), liquid | 5.26 |
| Dipole moment in benzene (25°C), D | 1.18 |
| Flash point (closed cup), °C | -57 |
| Ignition temperature in air, °C | 400 |
| Lower explosion limit in air, vol % | 2.8 |
| Upper explosion limit in air, vol % | 14.4 |
2. Chemical Reactions of Dimethylamine
Dimethylamine is basic, and consequently, it reacts with water and acids to form dimethylammonium compounds. Due to the presence of electron-donating methyl groups, dimethylamine possesses greater basicity than ammonia.
These methyl groups stabilize the positive charge formed during protonation. However, its basicity remains weaker compared to hydroxide and alkoxide ions.
Dimethylamine behaves as a nucleophile because of the presence of an unshared pair of electrons on the nitrogen atom. Dimethylamine readily reacts with a variety of substrates, including carboxylic acids, acyl halides, anhydrides, esters, lactones, isocyanates, α,β-unsaturated nitriles and esters, epoxides, alkyl halides, carbon dioxide, and carbon disulfide.
The reaction of dimethylamine with an organic acid or the ester of an organic acid leads to the formation of a dimethyl-substituted amide.
Dimethylamine and phosgene react to produce tetramethylurea. Reaction with urea leads to the expected N,N-dimethylurea.
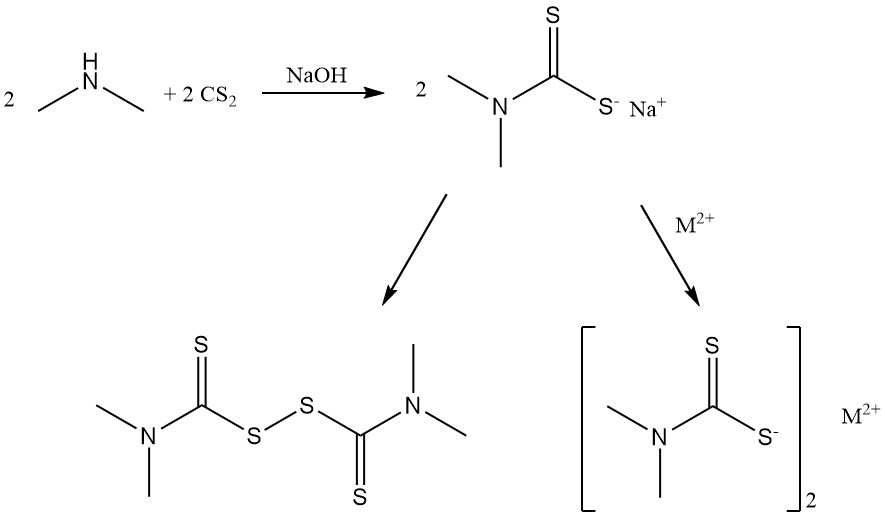
Reaction of dimethylamine with carbon disulfide produces dimethyldithiocarbamate salts and bis(dimethyldithiocarbamoyl) disulfide used as rubber accelerators.
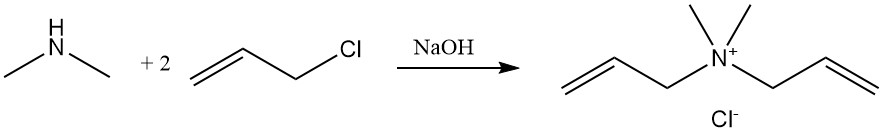
The reaction of dimethylamine with allyl chloride produces diallyldimethylammonium chloride, a monomer that is polymerized and used as a flocculant in water treatment applications.
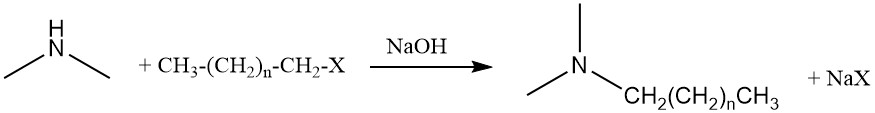
Dimethylamine can also undergo a further amination reaction with fatty alcohols or fatty halides to produce alkyldimethylamines, which are precursors to amine oxide surfactants.
Both nitrous acid and nitrosyl chloride convert dimethylamine to the corresponding nitrosamine.
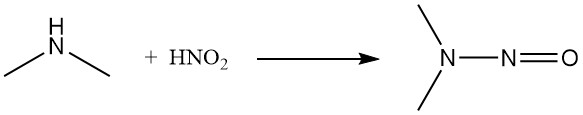
Ethylene oxide reacts with dimethylamine to give dimethylethanolamine.

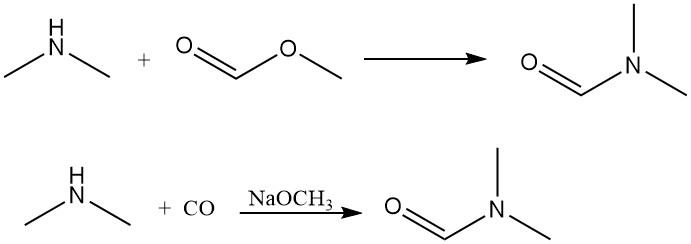
Dimethylamine reacts with methyl formate to generate dimethylformamide, a dipolar aprotic solvent. This reaction can also be achieved efficiently via direct carbon monoxide addition with a base as a catalyst.
Dimethylamine reacts with carbon dioxide to form dimethylamine carbamates under anhydrous conditions and dimethylamine carbonates in the presence of water.
Dimethylamine is corrosive to aluminum, copper, copper alloys, galvanized metal, magnesium, zinc, and zinc alloys. Anhydrous dimethylamine and its aqueous solutions can be handled by using materials constructed of steel.
3. Production of Dimethylamine
Commercially, dimethylamine is produced by the amination of methanol with ammonia. This process occurs in the vapor phase (300–500 °C and 790–3550 kPa) over fixed-bed reactors, yielding a mixture of mono-, di-, and trimethylamine.

Method 1: Acid-Catalyzed Methanol Amination (Leonard Process)
This traditional method uses a solid acid catalyst, typically amorphous silica-alumina, to promote the high-temperature conversion of methanol. The reaction reaches equilibrium, favoring trimethylamine formation at lower ammonia-to-methanol ratios. However, market demands prioritize dimethylamine, followed by methylamine and trimethylamine.
Method 2: Shape-Selective Acid-Catalyzed Methanol Amination
This method addresses the selectivity challenges of Method 1 by employing shape-selective zeolite catalysts (e.g., modified mordenites, RHO, and chabazite). These catalysts limit the formation of bulky trimethylamine molecules within their pores, leading to a product mixture enriched in dimethylamine.
Methanol amination involves sequential substitution reactions, transforming methanol into primary, secondary, and tertiary amines. Additionally, disproportionation reactions occur, establishing an equilibrium between the different methylamine products.
Method 1 typically operates at high methanol conversion and relies on rapid disproportionation reactions. This results in an equilibrium mixture governed by the ammonia-to-methanol ratio. To achieve the desired product distribution, excess unreacted methylamine and trimethylamine are often recycled, which is energy-intensive.
The advent of shape-selective zeolite catalysts offered a solution to optimize product selectivity. Due to their pore size limitations, these catalysts restrict the formation and diffusion of trimethylamine molecules, favoring the production of dimethylamine.
Examples of commercial processes include the following:
- Mitsubishi Rayon Process: This two-reactor setup uses a non-selective catalyst for initial trimethylamine disproportionation, followed by a shape-selective zeolite catalyst bed for further product adjustment.
- Mitsui Chemical Process: This process continuously recycles trimethylamine as an azeotropic mixture with ammonia, promoting its disproportionation and subsequent conversion to the desired dimethylamine using a shape-selective silylated mordenite catalyst.
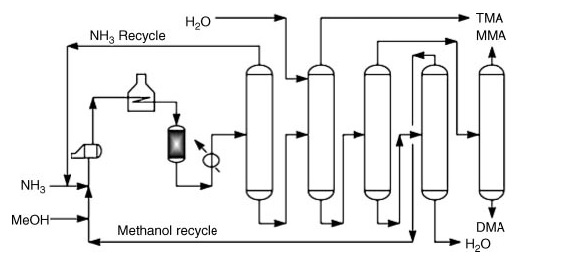
*MMA: methylamine; DMA: dimethylamine and TMA: trimethylamine
Following the reaction stages, pure product recovery involves a series of distillation steps:
- Ammonia separation and recycling.
- Trimethylamine recovery using water for enhanced volatility.
- Separation of methylamine and dimethylamine from water and unreacted methanol.
- Individual recovery of purified methylamine and dimethylamine.
- Separation of unreacted methanol from water.
4. Uses of Dimethylamine
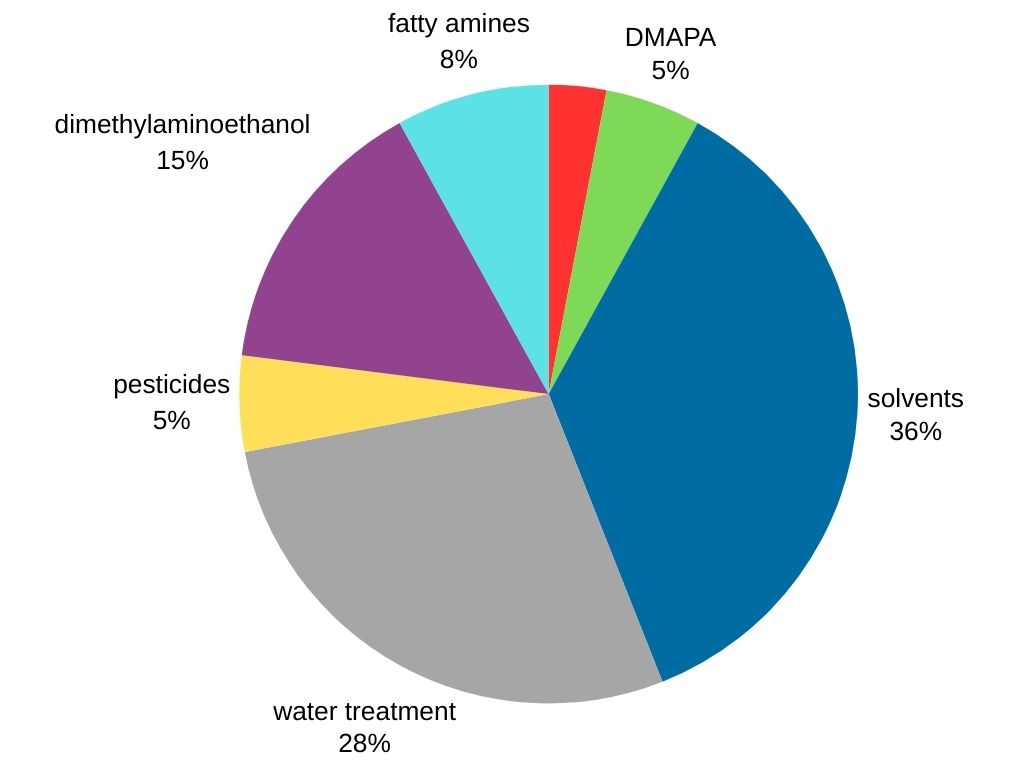
Dimethylamine stands out as the most demanded methylamine in the global market. It is used in various industries, including:
- Solvent Production: Dimethylamine serves as a crucial precursor for the synthesis of popular solvents like dimethylformamide and dimethylacetamide.
- Water Treatment
- Surfactants: Dimethylamine is used in the production of 3-dimethylaminopropylamine (DMAPA), which is a surfactant intermediate.
- Rubber Processing: Dimethylamine reacts with carbon disulfide to yield dimethyl dithiocarbamate, a crucial component in rubber vulcanization, which strengthens and enhances rubber properties.
- Agrochemicals (pesticides)
- Other chemical products (dimethylaminoethanol and fatty amines).
5. Toxicology of Dimethylamine
Dimethylamine is a flammable liquid or gas that presents a significant fire hazard.
Acute exposure effects
- Skin and eye irritants and corrosives: Contact can cause severe burns and irritation.
- Inhalation irritant: Breathing irritates the nose, throat, and lungs. High concentrations can cause fluid buildup in the lungs (pulmonary edema), a medical emergency.
Chronic exposure effects
- May damage the liver with repeated exposure.
- Potential reproductive hazard (effects on male testes).
- May irritate the lungs, leading to bronchitis with coughing, phlegm, and shortness of breath.
Dimethylamine is not classified as a carcinogen, according to current data.
Workplace Exposure Limits
- OSHA PEL (permissible exposure limit): 10 ppm averaged over an 8-hour workday.
- NIOSH REL (recommended exposure limit): 10 ppm averaged over a 10-hour workday.
- ACGIH TLV (threshold limit value): 5 ppm averaged over an 8-hour workday, 15 ppm short-term exposure limit (STEL).
Precautionary Measures
- Engineering controls: Prioritize enclosing operations and using local exhaust ventilation to minimize airborne exposure.
- Work practices: Maintain good hygiene practices, avoid skin and eye contact, and don’t eat, drink, or smoke in contaminated areas.
- Personal protective equipment: When engineering controls are insufficient, wear appropriate chemical-resistant gloves, clothing, safety glasses or goggles, and respirators following established protocols.
Storage and Handling
- Store in tightly sealed containers in a cool, well-ventilated area away from heat sources and incompatible chemicals (e.g., oxidizing agents, strong acids, and mercury).
- Ground and bond metal containers during transfer operations.
- Use non-sparking tools and equipment, especially when handling containers.
References
- Methylamines; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a16_535.pub4
- Methylamines; Kirk-Othmer Encyclopedia of Chemical Technology. – https://onlinelibrary.wiley.com/doi/10.1002/0471238961.1305200820211818.a01.pub2
- https://nj.gov/health/eoh/rtkweb/documents/fs/0737.pdf

