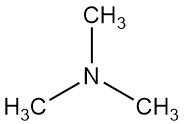
Trimethylamine is an organic compound with the formula N(CH3)3. It is a colorless gas or compressed liquid with a strong fishy odor at low concentrations. At higher concentrations, the odor becomes more ammonia-like.
Trimethylamine is a naturally occurring compound found in small amounts in many plants and animals. It is also produced by the breakdown of organic matter, such as fish and other seafood. In the human body, it is produced by the gut bacteria as they break down certain foods.
Table of Contents
1. Physical Properties of Trimethylamine
Trimethylamine has a stronger fishy odor than the other methylamines (methylamine and dimethylamine). The human nose can detect trimethylamine at a low concentration of <10 ppb produced from the decomposition of plants and animals.
Crystalline hydrate (CH3)3N·10 H2O is formed by cooling saturated aqueous solutions of trimethylamine. At atmospheric pressure, it forms minimum-boiling azeotropes with ammonia and other methylamines.
Triethylamine is soluble in various organic solvents, including methanol, ethanol, dimethylformamide, and ethylene glycol, and its aqueous solutions can dissolve many inorganic and organic compounds.
Some physical properties of trimethylamine are listed in Table 1.
| Property | Value |
|---|---|
| Molecular weight, g/mol | 59.11 |
| Boiling point (101.33 kPa), °C | 2.8 |
| Melting point, °C | -117.1 |
| Density (at 25 °C, liquid), g/cm3 | 0.6331 |
| pKa (at 25 °C) | 9.80 |
| Refractive index at 0 °C | 1.3631 |
| Surface tension (25 °C), 10-3 N/m | 13.47 |
| Heat of vaporization at 25 °C, kJ/mol | 22.864 |
| Heat of vaporization at boiling point, kJ/mol | 27.708 |
| Heat of fusion, kJ/mol | 6.548 |
| Standard heat of formation at 25 °C (liquid), kJ/mol | -45.80 |
| Standard heat of formation at 25 °C (gas), kJ/mol | -23.86 |
| Heat of combustion, standard state at 25 °C, liquid, kJ/mol | -2422.60 |
| Heat capacity, ideal gas (25 °C), J K-1 mol-1 | 91.8 |
| Critical temperature, °C | 160.1 |
| Critical pressure, MPa | 4.07 |
| Dielectric constant (25 °C), liquid | 2.44 |
| Dipole moment in benzene (25 °C), D | 0.87 |
| Flash point (closed cup), °C | -71 |
| Ignition temperature in air, °C | 190 |
| Lower explosion limit in air, vol % | 2 |
| Upper explosion limit in air, vol % | 11.6 |
2. Chemical Reactions of Trimethylamine
Trimethylamine is a weak base that forms salts like trimethylammonium chloride with hydrochloric acid. This reaction involves the lone pair of electrons on the nitrogen atom.
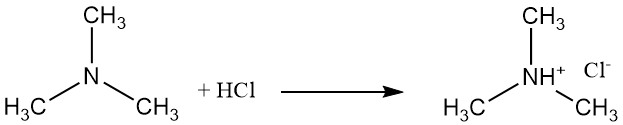
In the gas phase, the basicity of the methylamines increases based on methyl group substitution, as follows:
(CH3)3N > (CH3)2NH > CH3NH2 > NH3
However, in aqueous solution, the basicity of trimethylamine is lower than that of dimethylamine and methylamine. This is attributed to poorer solvation of the trimethylammonium ion, (CH3)3NH+, which has only one hydrogen available for hydrogen bonding with water molecules.
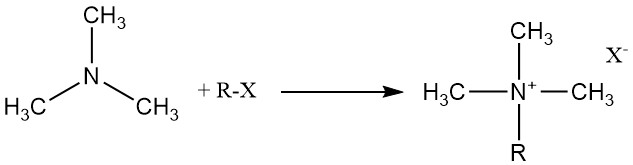
Trimethylamine reacts with organic and inorganic acids, alkyl halides, and epoxides to form quaternary ammonium salts.
The reaction of trimethylamine with ethylene oxide produces choline base or choline chloride when trimethylammonium chloride is used.

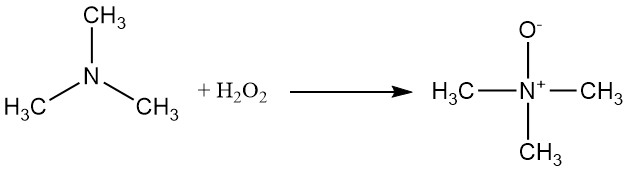
Trimethylamine can be oxidized with hydrogen peroxide or a peracid to yield trimethylamine oxide.
3. Industrial Production of Trimethylamine
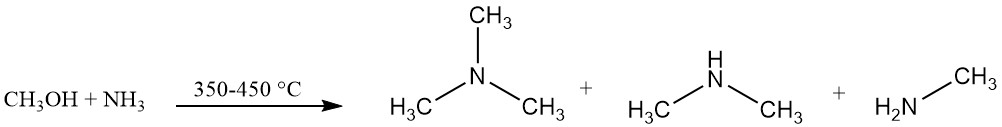
Trimethylamine is industrially produced by the reaction of ammonia and methanol in the presence of a catalyst, typically an alumina-based catalyst, at 350–450 °C. This reaction produces trimethylamine along with dimethylamine and methylamine.
The reaction equilibrium favors trimethylamine formation, but the market demand is higher for monoethylamine and especially dimethylamine.
The use of amorphous solid acid catalysts, including aluminas, silicas, and phosphates, favors the formation of trimethylamine in contrast to shape-selective zeolites that are selective for dimethylamine production.
The resulting mixture contains unreacted materials (ammonia, methanol) along with the desired products (monoethylamine, dimethylamine, and trimethylamine), water generated during the reaction, and other byproducts.
A series of four to five distillation columns separate and purify the individual components. Pure anhydrous trimethylamine is recovered from the mixture by extractive distillation with water to break the azeotropic mixture formed with the other methylamines.
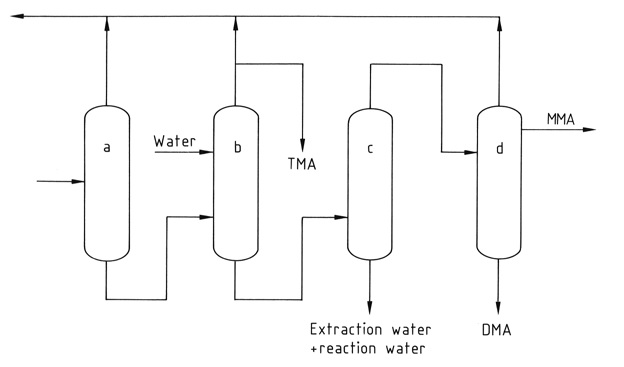
a) Ammonia column; b) Extractive distillation column; c) Column for water removal; d) MMA and DMA product column
*MMA: methylamine; DMA: dimethylamine; TMA; trimethylamine
4. Uses of Trimethylamine
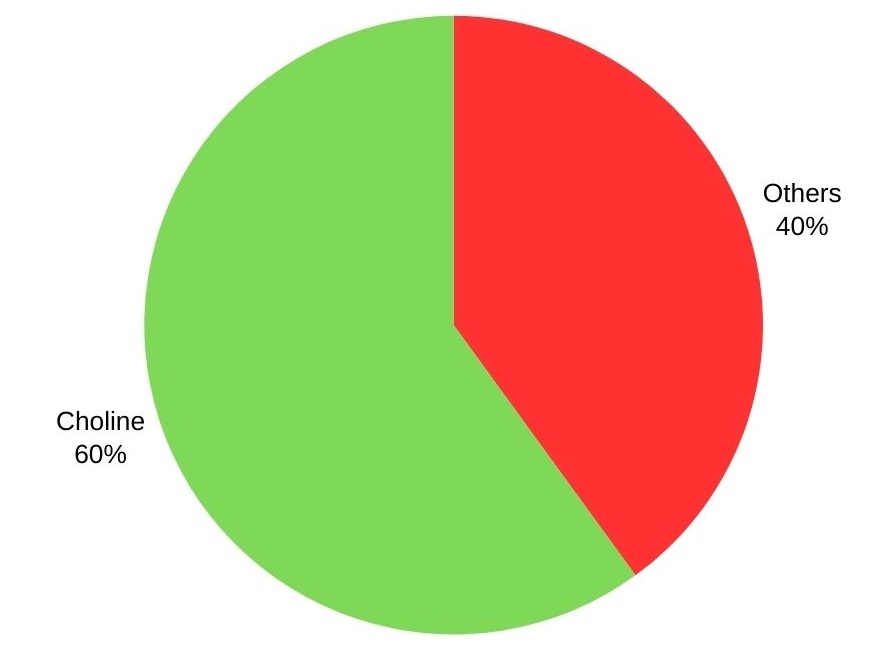
Trimethylamine is mainly used to produce choline and its salts, with over 60%. Other applications include the manufacturing of cationic starches, disinfectants, flotation agents, intense sweeteners, and ion-exchange resins.
Trimethylamine is used as a catalyst in the production of quaternary ammonium hydroxides and salts.
It can also be used to attract insects, and it is added to odorless natural gas as a safety measure to detect leaks.
5. Toxicology of Trimethylamine
Trimethylamine is a highly flammable liquid or gas irritant to the skin, eyes, and respiratory tract and causes pulmonary edema (fluid buildup in the lungs).
Exposure Limits
- National Institute for Occupational Safety and Health (NIOSH): 10 ppm (average) and 15 ppm (short-term)
- American Conference of Governmental Industrial Hygienists (ACGIH): 5 ppm (average) and 15 ppm (short-term)
Health Effects
- Acute (short-term): skin and eye irritation, respiratory tract irritation, coughing, shortness of breath. Severe exposure can lead to pulmonary edema.
- Chronic (long-term): There is no conclusive evidence for carcinogenicity or reproductive effects. Other long-term health effects are not yet fully characterized.
Handling and Storage
- Store away from oxidizing agents, mercury, ethylene oxide, strong acids, metals, and strong bases.
- Maintain a cool, well-ventilated storage area.
- Eliminate ignition sources like smoking and open flames.
- Ground and bond metal containers during transfer.
- Use non-sparking tools and equipment.
References
- Methylamines; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a16_535.pub4
- Methylamines; Kirk-Othmer Encyclopedia of Chemical Technology. – https://onlinelibrary.wiley.com/doi/10.1002/0471238961.1305200820211818.a01.pub2
- https://nj.gov/health/eoh/rtkweb/documents/fs/1927.pdf