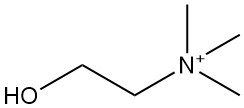
Choline [62-49-7], also known as trimethyl(2-hydroxyethyl)ammonium hydroxide, is a quaternary ammonium compound with the molecular formula C5H15O2N. It was first isolated from pig bile in 1849 by Stecker and is subsequently found in brain tissue.
The chemical structure of choline can be represented as (CH3)3N+CH2CH2OH∙OH–. Free choline exists in small quantities within biological materials. More frequent forms include phosphorylcholine, phosphatidylcholine (lecithin), and acetylcholine.
Table of Contents
Choline plays a role in the metabolism and synthesis of glycine, betaine, cysteine, serine, methionine, and various other methyl-containing biological compounds.
Choline has four primary functions in the body:
- Structural Component: As phosphatidylcholine (lecithin) and sphingomyelin, choline forms an important component of cell membranes.
- Lipotropic Agent: Choline prevents fatty liver disease.
- Neurotransmitter: Acetylcholine, a derivative of choline, acts as a neurotransmitter.
- Methyl Group Donor: Choline serves as a source of labile methyl groups.
While historically referred to as a vitamin, choline does not strictly meet the definition due to its lack of function as an enzymatic cofactor. Similar to vitamins, however, choline plays a vital role in nutrition. Daily intake recommendations are significantly higher for choline compared to vitamins (see Table 1).
| Population | Category | Adequate Intake (mg/day) |
|---|---|---|
| Infants | 0 – 5 months | 125 |
| Infants | 6 – 11 months | 150 |
| Children and adolescents | 1 – 3 years | 200 |
| 4 – 8 years | 250 | |
| 9 – 13 years | 375 | |
| Males (14 – 18 years) | 550 | |
| Females (14 – 18 years) | 400 | |
| Adults | Males (19 years and older) | 550 |
| Females (19 years and older) | 425 | |
| Pregnancy | All ages | 450 |
| Lactation | All ages | 550 |
The United States Pharmacopeia is developing monographs for choline chloride and choline bitartrate for inclusion in USP 24 supplements.
Fatty liver and reduced growth rate are the primary signs of choline deficiency in developing animals. Studies have shown that various species, including rats, mice, and poultry, require choline for optimal growth and healthy liver function.
Choline salts (chloride, dihydrogen citrate, and bitartrate) are frequently added to animal feed as a dietary supplement.
1. Properties of Choline
Choline is a basic compound with a pKa of 5.06 that can absorb carbon dioxide and water vapor from the atmosphere. Commercially available choline typically comes as solutions in methanol or water, with concentrations generally not exceeding 45% by volume.
Solubility:
- Soluble in ethanol
- Slightly soluble in acetone and chloroform
- Insoluble in ether, benzene, toluene, and carbon tetrachloride
Choline readily reacts with acids to form stable salts. However, crystallization is challenging for most choline salts except the chloride, bitartrate, and dihydrogen citrate forms. Additionally, choline can react with acids to form esters.
As a quaternary ammonium hydroxide, choline undergoes self-decomposition upon heating. This reaction, known as the Hofmann elimination, proceeds as follows:

The unstable vinyl alcohol intermediate can either rearrange to acetaldehyde or react with water to form ethylene glycol. Acetaldehyde can undergo further aldol condensation, leading to colored products. The liberated trimethylamine imparts a strong “fishy” odor to the solution.
2. Choline Salts
Choline forms various salts with different physical and chemical properties. Here’s a description of some common choline salts:
2.1. Choline Chloride [67-48-1]
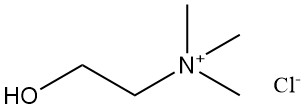
- Formula: C5H14ONCl
- Molar Mass: 139.63 g/mol
- Appearance: A white, crystalline solid with a slight amine-like odor and a strong brackish taste.
- Solubility:
- It is very soluble in water
- Freely soluble in alcohol
- Slightly soluble in acetone and chloroform
- Almost insoluble in ether and benzene
- Properties: Hygroscopic; decomposes around 180°C.
- pH: neutral to litmus paper.
2.2. Choline Hydrogen Tartrate [87-67-2]
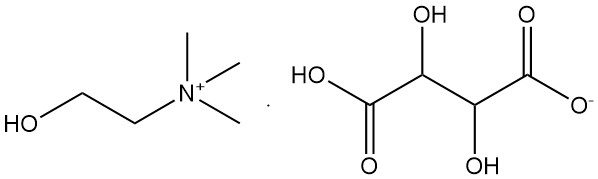
- Formula: C9H19O7N
- Molar Mass: 253.26 g/mol
- Melting Point: 149 – 153 °C
- Appearance: white crystalline powder with an acidic taste and a slight amine odor.
- Solubility:
- It is very soluble in water
- Slightly soluble in alcohol
- Almost insoluble in ether, benzene, and chloroform
- pH: A 25% aqueous solution has a pH of approximately 3.5.
2.3. Choline Dihydrogen Citrate [77-91-8]
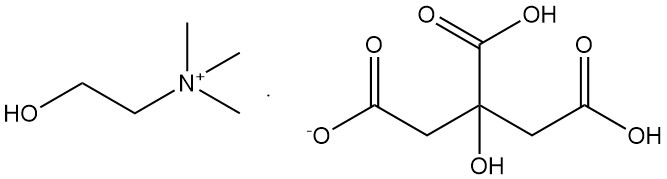
- Formula: C11H21O8N
- Molar Mass: 295.30 g/mol
- Melting Point: 105 – 107.5 °C
- Appearance: white granular to fine-crystalline powder with a faint amine-like odor and an acidic taste.
- Solubility:
- Hygroscopic and very soluble in water
- Soluble in alcohol
- Almost insoluble in ether, benzene, and chloroform
- pH: A 25% aqueous solution has a pH of approximately 4.3.
2.4. Choline Bicarbonate [78-73-9]
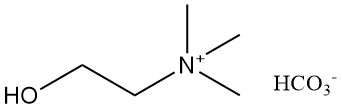
- Formula: C6H15NO4
- Molar Mass: 165.2 g/mol
- Appearance: Clear liquid, colorless to slightly yellow, with a characteristic amine-like odor.
- Solubility:
- It is very soluble in water
- Freely soluble in alcohol
- Slightly soluble in benzene
- pH: The aqueous solution (75% choline bicarbonate) has a pH between 9.0 and 11.5.
2.5. Choline Gluconate [608-59-3]
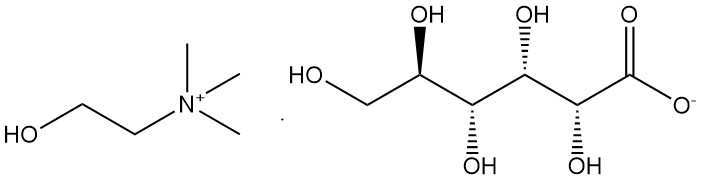
- Formula: C11H25O8N
- Molar Mass: 299.33 g/mol
- Appearance: hygroscopic yellow mass.
- Solubility:
- Soluble in water
- Slightly soluble in ethanol
3. Production of Choline
Choline is primarily produced commercially through the reaction of trimethylamine, ethylene oxide, and water. This process can be adapted to yield choline salts by incorporating an acid during the reaction. Production typically occurs in either batch or continuous manufacturing setups.
Alternative synthesis methods exist for choline, but this specific reaction remains the dominant industrial process due to its efficiency and scalability.
Choline chloride can be produced by the reaction of trimethylamine with chlorohydrin.
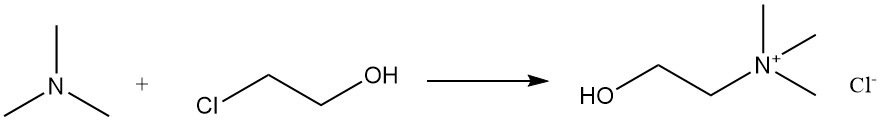
4. Uses of Choline
4.1. Animal Nutrition
Choline deficiency in domesticated animals is well-understood and can be effectively prevented through dietary supplementation with choline salts.
4.2. Human Therapeutics
Research in human nutrition explores the potential of choline to alleviate various diseases. While many investigations are in the early stages, they hold promise for a deeper understanding of choline’s role in human health.
Choline salts like chloride, hydrogen tartrate, dihydrogen citrate, and gluconate are used therapeutically in patients with liver cirrhosis (non-fibrotic stage). Oral choline administration appears effective in reversing fatty liver infiltration and halting the progression of cirrhosis.
Treatment typically involves a low-fat, high-protein, high-calorie diet supplemented with vitamins and choline. Additionally, oral choline salts can be used to meet the recommended daily intake for humans.
4.3. Pharmacological Applications
Several choline derivatives possess distinct pharmacological properties:
- Acetylcholine is used as a vasodilator.
- Methacholine chloride functions as a parasympathetic stimulant and anti-epinephrine substance.
- Carbamoylcholine chloride enhances peripheral circulation in cases of vasospasms caused by peripheral vascular disorders.
4.4. Industrial Applications
Growing demand for safe and effective surface-active agents has attracted attention to choline in the food and cosmetic industries. Acylcholine compounds, such as stearoylcholine chloride, have been proposed as ingredients in hair conditioners.
4.5. Agricultural Applications
The choline derivative chlorocholine chloride is used in agriculture as a plant growth regulator, promoting plant compactness in contrast to the growth-accelerating effects of gibberellic acid. It is used for ornamental plants like poinsettias and azaleas.
It is also used to prevent the lodging and wind-related flattening of wheat crops and in greenhouses to prevent etiolation (elongation due to insufficient light) in tomatoes, leading to earlier and larger fruit yields.
5. Toxicology of Choline
Choline exhibits low inherent toxicity. Studies have shown that oral administration of choline chloride in rats resulted in an LD50 (lethal dose, 50%) value between 3-6 grams per kilogram of body weight. However, due to its strong basic character, choline can be corrosive to tissues upon direct contact. This necessitates handling precautions to avoid skin and eye irritation.
References
- Choline; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a07_039
- Choline; Present Knowledge in Nutrition, Tenth Edition. – https://onlinelibrary.wiley.com/doi/10.1002/9781119946045.ch26
- Choline; Kirk-Othmer Encyclopedia of Chemical Technology. – https://onlinelibrary.wiley.com/doi/10.1002/0471238961.0308151207090404.a01
- Choline: an essential nutrient for public health. – https://onlinelibrary.wiley.com/doi/10.1111/j.1753-4887.2009.00246.x


