Maleic Anhydride: Properties, Reactions, Production and Uses
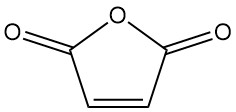
What is Maleic Anhydride?
Maleic anhydride, also known as 2,5-furandione, is an organic compound with the formula C4H2O3. It is a colorless or white solid with a strong, irritating odor that is industrially more important compared to maleic acid.Table of Contents
1. Physical Properties of Maleic Anhydride
Maleic anhydride forms orthorhombic needle crystals. It is soluble in water, acetone, ethanol, xylene, ethyl acetate, chloroform, benzene, toluene, tetrachloromethane, and many other organic solvents.
Maleic anhydride is hygroscopic and flammable and can form explosive mixtures with air. It can sublime. The pH of maleic anhydride water solutions is 2.42 at 1×10-2 M, 2.62 at 5×10-3 M, and 3.10 at 1×10-4 M.
The most important physical properties of maleic anhydride are summarized in the following table.
| Property | Value |
|---|---|
| CAS number | 108-31-6 |
| Chemical formula | C4H2O3 |
| Molecular mass | 98.06 g/mol |
| Melting point | 52.85 °C |
| Boiling point (101.3 kPa) | 202.0 °C |
| Density | 1.48 g/cm3 |
| Vapor Density | 3.40 |
| Heat of combustion | -1391.2 kJ/mol |
| Specific heat (liquid) | -1.67 kJ mol-1 K-1 |
| Heat of evaporation | 54.8 kJ/mol |
| Heat of fusion | 13.66 kJ/mol |
| Flash Point | 102 °C (closed cup) 110 °C (open cup) |
| Autoignition Temperature | 477 °C |
2. Reactions of Maleic Anhydride
Maleic anhydride exhibits high reactivity due to the presence of a double bond and its anhydride group.
Maleic anhydride readily undergoes hydrolysis with water to form maleic acid. This reaction is exothermic.
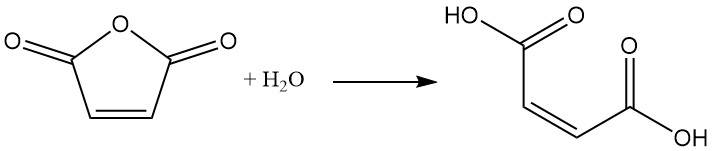
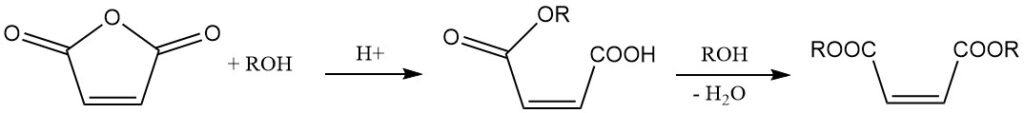
Reaction with alcohols in the presence of a catalyst leads to the formation of maleic acid esters. The choice of catalyst and reaction temperature determine the product. At lower temperatures, semi-esters are formed, while higher temperatures favor diester formation with water elimination.
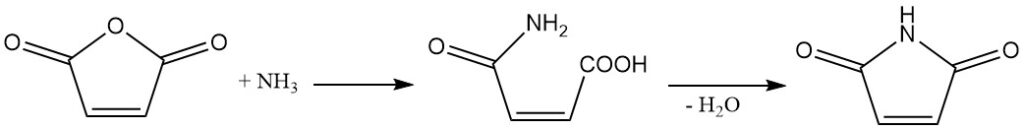
Maleic anhydride reacts with ammonia or amines to produce the corresponding semiamides. Further dehydration leads to the formation of cyclic imides.
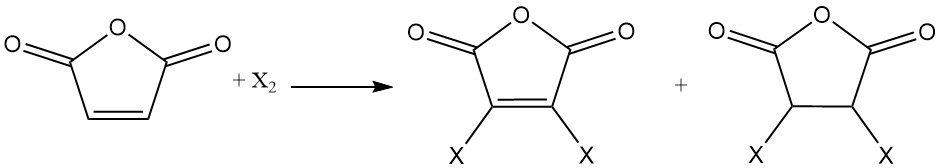
The double bond of maleic anhydride undergoes addition reactions with halogens. Depending on reaction conditions, mono- or dihalogenated maleic anhydrides or dihalogenated succinic anhydrides can be formed.
The hydrogenation of maleic anhydride yields various products, depending on the reaction conditions. These products include succinic anhydride, 1,4-butanediol, tetrahydrofuran, or butyrolactone.

The addition of olefins leads to the formation of alkenylsuccinic anhydrides.
Maleic anhydride participates in Diels-Alder reactions with conjugated dienes.
Maleic anhydride can undergo homopolymerization and copolymerization reactions.
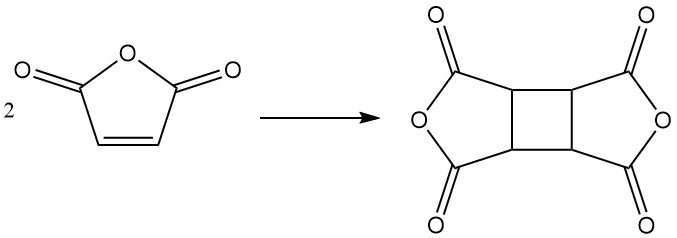
Under specific conditions, maleic anhydride can dimerize to form cyclobutane tetracarboxylic dianhydride (CBTA), which is used in the production of polyimides.
3. Production of Maleic Anhydride
Maleic anhydride is produced by the catalytic oxidation of suitable hydrocarbons in the gas phase. Traditionally, benzene was the primary feedstock. However, C4 hydrocarbons (like butane) have become increasingly important in recent years.
3.1. Production of Maleic Anhydride by Oxidation of Benzene
To produce maleic anhydride (Figure 1), benzoene is preheated and mixed with an air stream to form a homogenous mixture. Tubular reactors with vertical tubes packed with a catalyst such as vanadium and molybdenum oxides on an inert carrier are used.
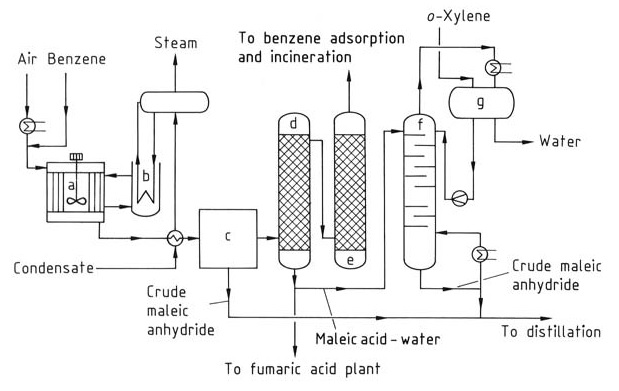
a) Reactor; b) Salt bath cooler; c) Partial condenser; d) Acid scrubber; e) Alkali scrubber; f ) Dehydration column; g) Phase separator
The reaction is exothermic, requiring control over operating temperature and a pressure between 0.15-0.25 MPa to optimize maleic anhydride yield and minimize combustion to CO2 and CO. Heat generated during the reaction (around 27 MJ per ton of benzene) is removed using circulating molten salts, which are subsequently cooled with water.
The following reaction represents maleic anhydride production:
C6H6 + 4.5 O2 → C4H2O3 + 2 CO2 + 2 H2O (ΔH = -1875 kJ/mol)
However, some undesirable combustion also occurs. Unreacted benzene can be recovered using techniques like adsorption and reused in the process. While patents exist for processes that recycle a larger portion of the reaction gas after maleic anhydride separation, these haven’t been widely adopted commercially yet.
Separation of crude maleic anhydride
The reactor effluent is initially cooled to prevent the condensation of maleic anhydride and water. Two methods can then be used for further processing:
- Partial condensation: The gas mixture is cooled to around 55 °C, allowing maleic anhydride to condense and be separated as a liquid. However, prolonged contact with the water-containing gas can lead to maleic acid formation. This approach typically recovers 40–60% of the maleic anhydride.
- Water scrubbing: This method captures all the maleic anhydride in the reaction gas as a maleic acid solution. However, the subsequent dehydration step to recover maleic anhydride is energy-intensive and is only preferred for reaction gases with high water content (like those from C4 oxidation).
3.2. Production of Maleic Anhydride by Dehydration of Aqueous Maleic Acid Solutions
Maleic anhydride can be derived from the maleic acid solutions obtained by scrubbing the reaction gas with water.
At temperatures exceeding 150 °C, even trace amounts of alkali in the wash solution can cause the decarboxylation of maleic anhydride. Therefore, alkali-free wash water is necessary.
Dehydration of maleic acid requires temperatures above 100 °C (ΔH = +34.88 kJ/mol). However, achieving an industrially relevant reaction rate necessitates temperatures exceeding 130 °C. This higher temperature system also promotes the undesired isomerization of maleic acid to fumaric acid.
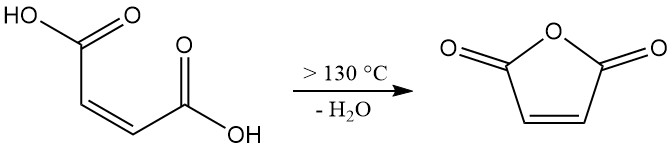
While fumaric acid itself doesn’t decompose significantly to form maleic anhydride below 230 °C, its presence reduces the overall yield of the desired product. To minimize isomerization, a short residence time at elevated temperatures is critical during the dehydration process.
Two main processes are used for dehydrating aqueous maleic acid solutions: the entraining agent method and the thermal process without an entraining agent.
3.2.1. Entraining Agent Method
In this process, the aqueous maleic acid solution is fed into the upper section of a distillation column, where it mixes with a boiling mixture of maleic anhydride and an entraining agent such as xylene.
The entrainer (xylene) forms an azeotropic mixture with water, allowing both to be removed from the column overhead as a vapor stream. This vapor stream is then separated in a decanter into organic and aqueous phases.
The organic phase containing the xylene is recycled back to the distillation column’s top section, while the aqueous phase is returned to the reaction gas scrubber of the oxidation plant.
The bottom product stream from the distillation column, containing maleic anhydride (10–40%), xylene (1–5%), maleic acid (1-3%), and fumaric acid (1-3%), undergoes further distillation to isolate pure maleic anhydride. This process can be operated continuously or in batches.
3.2.2. Thermal Process without Entraining Agent
This method uses thin-layer evaporation technology to achieve thermal dehydration of the aqueous maleic acid solution. The technique allows for efficient water removal as steam while minimizing the residence time of maleic acid at high temperatures (150–200 °C). This short exposure time helps suppress isomerization to fumaric acid (only 1–3%).
Multiple thin-layer evaporator units are often arranged in series. The liquid maleic anhydride obtained from the partial condenser can also be fed to the second stage of this system to dehydrate any remaining maleic acid (1–5%).
This continuous process produces high-purity maleic anhydride (99%) after a final distillation step. The bottom product from the last evaporator contains fumaric acid and high-boiling residues, which are discarded.
These continuously operating plants require periodic shutdowns for cleaning.
3.3. Production of Maleic Anhydride by Oxidation of C4 Hydrocarbons
In recent years, C4 hydrocarbon oxidation, primarily using n-butane or n-butane-n-butene mixtures rich in paraffins, has emerged as a significant alternative to traditional benzene oxidation for maleic anhydride production.
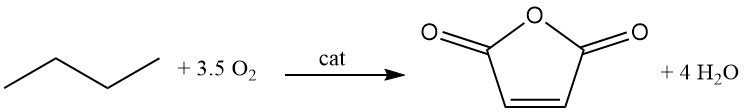
Different types of processes have been developed, such as fixed-bed, fluidized-bed, and transport-bed processes.
3.3.1. Fixed-Bed Process
The fixed-bed process is a commercially established method that uses vanadium-phosphorus oxide (V-P-O) catalysts packed in tubular reactors, similar to benzene oxidation. The reaction is highly exothermic.
C4H10 + 3.5 O2 → C4H2O3 + 4 H2O (ΔH = -1260 kJ/mol)
However, compared to benzene oxidation, lower conversion rates of 80% and selectivities (70%) are observed. Recovering unreacted C4 hydrocarbons is more challenging than with benzene.
3.3.2. Fluidized-Bed Process
The fluidized-bed process offers more uniform temperature control within the reactor, minimizing “hot spots” and potentially enhancing reaction selectivity. However, the fluidization process also involves intensive product remixing, which can counteract the benefit.
Another challenge is mechanical stress on the V-P-O catalyst due to abrasion and erosion. Despite these drawbacks, fluidized beds can operate at higher C4 concentrations (within the explosion range) due to their effectiveness as flame barriers.
The ALMA process (Alusuisse, Lummus) is an example of a fluidized-bed reactor coupled with a non-aqueous processing unit.
3.3.3. Transport-Bed Process
This process was developed by Monsanto and DuPont and uses two reactors. The V-P-O catalyst consumed in the C4 oxidation is regenerated with oxygen in the first reactor. The regenerated catalyst then reacts with n-butane in the second reactor under near-stoichiometric conditions with minimal atmospheric oxygen.
This process achieves high maleic anhydride formation selectivity (75 mol%) while minimizing product gas remixing, however, it is still under development.
Due to the increased water formation compared to benzene oxidation, direct liquefaction of maleic anhydride from C4 reaction gas via partial condensation is limited. A larger portion (65-70%) needs to be recovered as maleic acid by water scrubbing, followed by dehydration.
An alternative purification method uses organic solvent absorption of maleic anhydride from the reaction gas, which enables separation without significant maleic acid formation. The solvent-maleic anhydride mixture then undergoes fractional distillation to isolate pure maleic anhydride.
3.4. Purification of Crude Maleic Anhydride
There are two main methods for purifying crude maleic anhydride: batchwise distillation and continuous distillation.
3.4.1. Batchwise distillation
Batchwise distillation is used for processing directly separated maleic anhydride or mixtures obtained from entraining agent dehydration. The crude maleic anhydride is charged into a batch distillation column and initially heated under total reflux conditions. This achieves a complete dehydration of any remaining maleic acid.
The small amount of water eliminated during the dehydration condenses and is separated from the recirculating entraining agent (usually xylene). The xylene is then removed by distillation. Finally, the purified maleic anhydride is recovered by distillation under vacuum.
Batch stills with capacities ranging from 50 to 150 cubic meters and distillation columns with 10–20 trays are commonly used for this process.
3.4.2. Continuous distillation
Continuous distillation is more economical for large-scale maleic anhydride production facilities.
In this process (Figure 2), a continuous stream of the crude maleic anhydride mixture is fed to the distillation system. Residual xylene is first removed in a dedicated column (f). Finally, pure maleic anhydride is isolated from the top of a second column (g) via distillation.
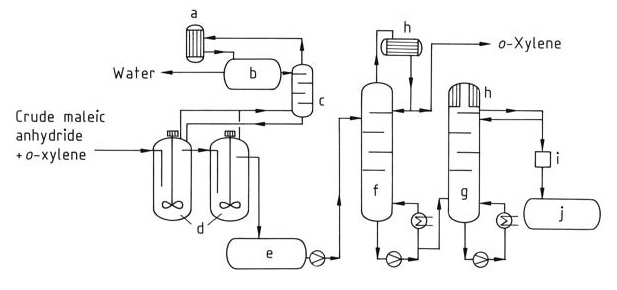
a) Condenser; b) Separating vessel; c) Column; d) Post dehydration; e) Intermediate tank; f ) o-Xylene column; g) Pure maleic anhydride column; h) Cooler; i) Colorimeter; j) Pure maleic anhydride vessel
4. Uses of Maleic Anhydride
Maleic anhydride’s unique properties as a dicarboxylic anhydride with a double bond make it a versatile building block for various industrial applications.
Maleic anhydride is mostly used for the production of polyesters and alkyd resins, which are used in the production of fiberglass-reinforced plastics for construction, electrical applications, pipelines, and marine construction. It is also used in the formulation of lubricants and plasticizers.
Maleic anhydride readily participates in copolymerization reactions. Some industrially important examples include maleic anhydride-styrene copolymers used for engineering plastics and maleic anhydride-acrylic acid copolymers used in the detergent industry.
Maleic anhydride undergoes Diels-Alder reactions with dienes like butadiene to form tetrahydrophthalic anhydride (1). Further hydrogenation yields hexahydrophthalic anhydride (2), which is a valuable curing agent for epoxy resins. A similar process using isoprene yields methylhexahydrophthalic anhydride for the same application.
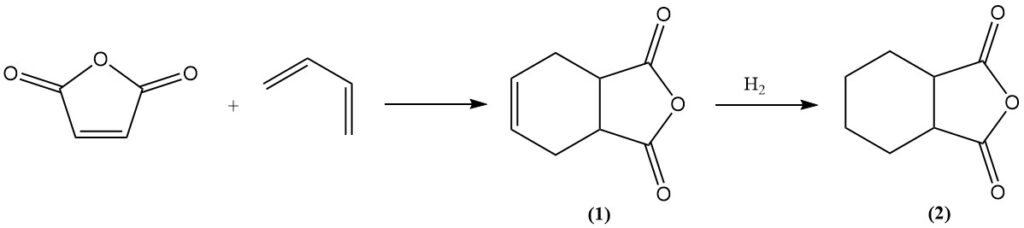
Smaller quantities of maleic anhydride are used in the production of pesticides like captan and malathion, as well as growth inhibitors such as maleic acid hydrazide.
Maleic acid esters can be transformed into surfactants by reactions with sodium hydrogen sulfite.
Maleic anhydride can be used as a drying accelerator when added to drying oils, improving the quality of lacquers.
5. Toxicology of Maleic Anhydride
Maleic anhydride can hydrolyze to maleic acid in water, so its toxicological properties are influenced by the presence of maleic acid.
Maleic anhydride shows moderate acute toxicity based on LD50 values (481 mg (rat, oral), 465 mg (mice, oral), 2620 mg (rabbit, percutaneous), and 390 mg (guinea pig, oral) per kilogram of body weight).
The primary acute toxic effect is its local irritant and corrosive effects on the skin, mucous membranes, and eyes. Exposure can cause irritation at concentrations as low as 1.5–2 ppm, with severe irritation occurring above 2.5 ppm.
Inhaling maleic anhydride at concentrations exceeding 1.2 ppm for extended periods may trigger asthmatic symptoms.
Maleic anhydride is a strong sensitizer, meaning repeated exposure can lead to allergic reactions in both humans and animals.
Animal studies haven’t shown evidence of carcinogenic, teratogenic (birth defects), or reproductive toxicity from maleic anhydride exposure. However, no systemic effects were observed only up to an exposure level of 2.4 ppm, so effects at higher concentrations are not entirely ruled out.
Occupational exposure limits have been established based on the irritant and sensitizing properties:
- MAK (German Commission for the Investigation of Health Hazards of Working Materials): 0.2 ppm
- TLV-TWA (American Conference of Governmental Industrial Hygienists Threshold Limit Value—Time-Weighted Average): 0.25 ppm
References
- Maleic and Fumaric Acids; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a16_053
- https://pubchem.ncbi.nlm.nih.gov/compound/Maleic-Anhydride
- Method for producing cyclobutane tetracarboxylic acid derivative. – https://patents.google.com/patent/WO2015108168A1/en
