Dichloroacetic Acid: Properties, Production and Applications

What is dichloroacetic acid?
Dichloroacetic acid is a derivative of acetic acid with the chemical formula CHCl2COOH. It is a highly corrosive liquid that emits acidic vapors, which can cause irritation to the mucous membranes.
Dichloroacetic acid is completely miscible with water and can dissolve readily in various organic solvents such as alcohols, ketones, hydrocarbons, and chlorinated hydrocarbons.
Table of Contents
1. Physical properties of Dichloroacetic Acid
The physical properties of dichloroacetic acid, also known as 2,2-dichloroethanoic acid, include:
- Molar mass: 128.95 g/mol
- Boiling point: 192 °C (101.3 kPa)
- Freezing point: 13.5 °C
- Density: 1.564
- Vapor pressure: 0.19 kPa (at 20 °C)
- Dissociation constant: 5×10-2 mol/L (at 18 °C)
2. Chemical Reactions of Dichloroacetic Acid
Dichloroacetic acid possesses two chlorine atoms that are susceptible to displacement reactions.
When reacted with aromatic compounds, it forms diaryl acetic acids, while in the presence of phenol, diphenoxy acetic acids are produced.
Compared to chloroacetic acid, dichloroacetic acid demonstrates lower susceptibility to hydrolysis. However, the presence of dichloroacetic acid impurity in chloroacetic acid can lead to cross-linking during the production of carboxymethyl cellulose (CMCs) and starches. The resulting cross-linking effect can be desirable or undesirable, depending on the intended application of the final product.
3. Production Methods of Dichloroacetic Acid
The most economically viable approach for producing dichloroacetic acid involves the hydrolysis of dichloroacetyl chloride.

Additionally, a 90% yield of 98% pure dichloroacetic acid can be achieved through the hydrolysis of pentachloroethane using 88–99% sulfuric acid or by oxidizing 1,1-dichloroacetone with nitric acid and air.
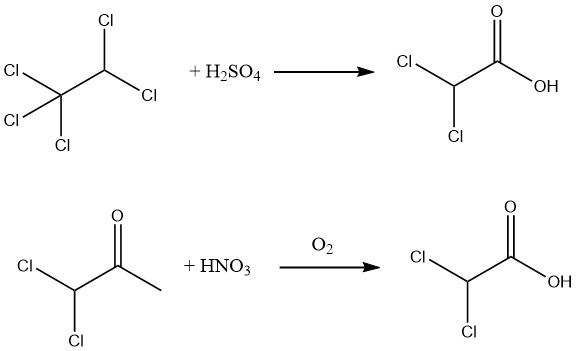
Hydrolysis of the methyl ester, readily obtainable through the esterification of crude dichloroacetic acid, allows for the production of exceptionally pure dichloroacetic acid.

Moreover, the catalytic dechlorination of trichloroacetic acid or ethyl trichloroacetate with hydrogen over a palladium catalyst provides a method for obtaining dichloroacetic acid and ethyl dichloroacetate.

Separation of pure dichloroacetic acid from other chloroacetic acids proves challenging through physical means such as fractional distillation, primarily due to the slight differences in boiling points, especially between di- and trichloroacetic acid.
However, effective distillation columns can satisfactorily fractionate ester mixtures. Additionally, mixtures of the salts derived from the three chloroacetic acids can be washed with water, alcohol, or water–alcohol solutions to preferentially dissolve dichloroacetate, which can then be acidified to obtain pure dichloroacetic acid.
In laboratory settings, dichloroacetic acid can be synthesized by reacting chloral hydrate with either potassium or sodium cyanide:
Cl3CCH(OH)2 + KCN → HCN + KCl + Cl2CHCOOH
4. Applications of Dichloroacetic Acid
Dichloroacetic acid finds utility as a test reagent in analytical measurements during fiber manufacture, specifically for poly(ethylene terephthalate) production. It also serves as a medicinal disinfectant, acting as a substitute for formalin.
Furthermore, dichloroacetic acid plays a vital role as a deblocking agent in the solid-phase synthesis of oligonucleotides. To fulfill this function, a high-purity dichloroacetic acid, substantially devoid of chloral (trichloroacetaldehyde), is required.
In organic synthesis, dichloroacetic acid, especially in the form of its esters, serves as a reactive starting material. It is utilized in the production of glyoxylic acid, dialkoxy and diaroxy acids, as well as sulfonamides, thus serving as a crucial intermediate compound.
Reference
- Chloroacetic Acids; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_537.pub3
