Chloroamines: Properties, Reactions and Examples

Chloroamines are nitrogen-containing compounds, either inorganic or organic, where one or more chlorine atoms are directly attached to a nitrogen atom. Monochloroamine (NH2Cl), discovered in the early 1800s, is a well-known example.
Chloroamines are chlorinating and oxidizing substances used in synthetic reactions and as bleaching agents, disinfectants, and bactericides. Compared to chlorine gas or metallic hypochlorites, they offer safer handling and greater stability.
Because of this, they are used in drinking water purification and swimming pool sanitation. N-chloroisocyanuric acids and 1-bromo-3-chloro-5,5-dimethylhydantoin are becoming more important in the sanitization and disinfection markets than calcium hypochlorite, 1,3-dichloro-5,5-dimethylhydantoin, and chloramine-T.
Table of Contents
1. Chemical Properties of N-Chloroamines
N-Chloroamines possess oxidizing capabilities, accepting two electrons to convert into chloride ions. This property allows them to oxidize hydriodic acid, releasing iodine, in a reaction used for quantitative analysis:
2 HI + RR’NCl → HCl + RR’NH + I2
The theoretical chlorine content in an N-chloroamine is calculated as twice the chlorine mass fraction. However, practical applications express it as the equivalent of elemental chlorine based on its actual oxidizing ability.
N-chloroamines undergo hydrolysis in water, releasing hypochlorous acid (HOCl). The quantitative hydrolysis constant (K value), ranging between 10-4 and 10-10 (Table 1), reflects this reaction:
RR’NCl + H2O ⇌ RR’NH + HOCl
K = [RR’NH][HOCl] / [RR’NCl]
| N-Chloroamine | K value |
|---|---|
| Trichloroisocyanuric acid | 6.7 × 10-4 |
| 1,3-Dichloro-5,5-dimethylhydantoin | 2.5 × 10-4 |
| N-Chlorosuccinimide | 6.6 × 10-5 |
| Dichloramine-T | 8.0 × 10-7 |
| Chloramine-T | 4.9 × 10-8 |
| Monochloroamine | 2.8 × 10-10 |
This K value relates to the bactericidal power of N-chloroamines, as their effectiveness depends on generating hypochlorous acid in water.
N-Cl bonds in N-chloroamines are covalent and readily hydrolyzed, releasing hypochlorous acid. They exhibit thermal instability, often failing to melt congruently and potentially exploding at high temperatures. Trichloroamine (NCl3) is a particularly unstable compound and can cause explosions even as an impurity.
Proper storage of N-chloroamines requires cool temperatures and protection from light, water, amines and ammonium compounds, strong acids and bases, and easily oxidized organic material.
2. Reactions of Chloroamines
Many N-chloroamines demonstrate valuable roles as reagents or intermediates in diverse organic reactions.
Hofmann Degradation: N-chloroamides derived from organic carboxylic acids undergo reduction with alkali to yield corresponding amines with high efficiency. This reaction, known as Hofmann degradation, is used for the synthesis of aromatic amines, heterocyclic amines, and alicyclic amines.
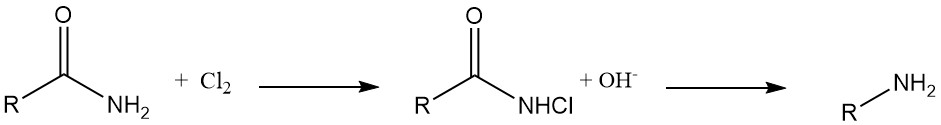
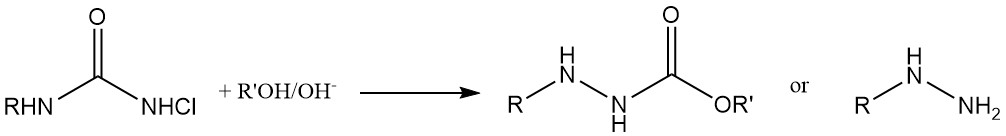
Hydrazine Synthesis: N-chloroureas transform into hydrazines upon treatment with a base in alcohol or water solutions.
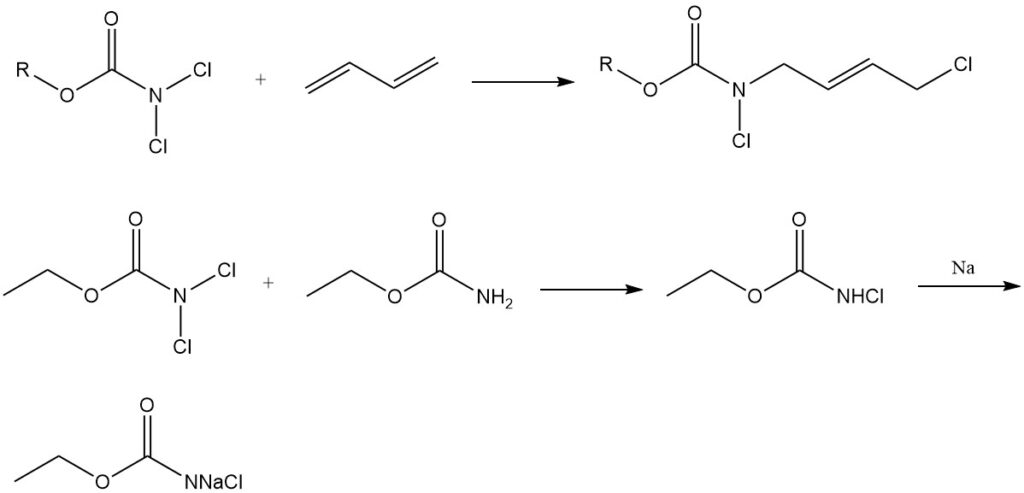
Chlorinating Agents and Diene Addition: N,N-Dichlorocarbamates (Cl2NCOOR) are used as chlorinating agents and also engage in addition reactions with dienes. They further act as starting materials for the synthesis of N-halo-N-metallocarbamidates, important intermediates in producing carbamate derivatives of physiologically active compounds.
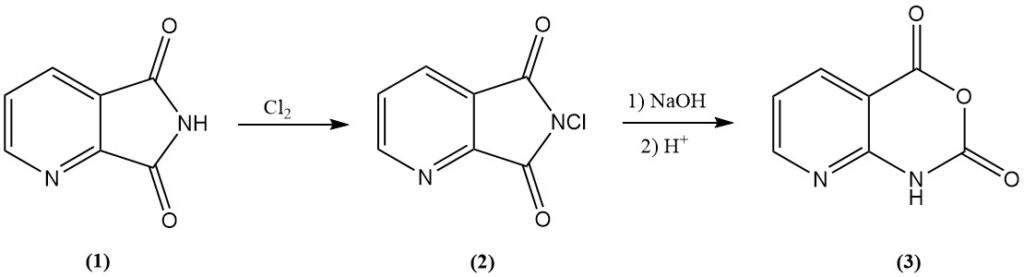
8-Azaisatoic Anhydride (3), an intermediate in various agrichemicals, is efficiently obtained by chlorinating 2,3-pyridinedicarboximide (1) to N-chloro-2,3-pyridinedicarboximide (2), followed by alkaline treatment.
Heterocyclic Ring Synthesis: N-chloroamidines and N-chloroguanidines are used as raw materials for the production of heterocyclic ring systems like imidazoles, thiazoles, and oxadiazoles.
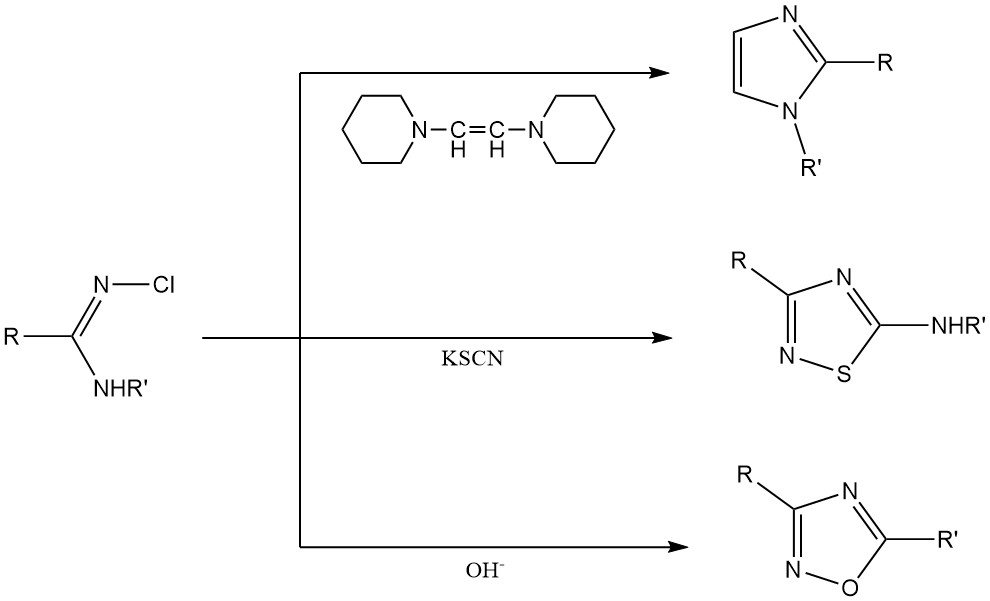
3. Inorganic N-Chloroamines
Monochloroamine (NH2Cl) is a colorless, strong-smelling liquid that is more stable in aqueous solutions than other N-chloroamines like dichloroamine (NHCl2) and trichloroamine (NCl3). Its primary application is in drinking water disinfection. It is also used as an oxidizing agent in organic synthesis, particularly for trisubstituted phosphines.
N-chloroamines are synthesized by controlled pH reactions between ammonium salts and hypochlorous acid, or chlorine.
Extreme caution is important when handling pure N-chloroamines due to their inherent instability and explosiveness, even at room temperature.
N-chlorosulfamic acid (ClSO2NH2), (N,N-dichlorosulfamic acid, and sodium N-chloroimidodi-sulfonate (ClSO2NNaSO2) are used as disinfectants and bleaching agents for paper or fabrics. They are produced from sulfamic acid and hypochlorous acid.
4. N-Chloroisocyanuric Acids
N-chloroisocyanuric acids (chloroisocyanurates), like trichloroisocyanuric acid (Symclosene or TCC) and sodium dichloroisocyanurate (DCC-Na), are witnessing a remarkable rise in their use as disinfectants. This increasing popularity is due to their superior stability and user-friendliness compared to traditional metal hypochlorites.
Table 2 lists some important physical properties of TCC and DCC-Na.
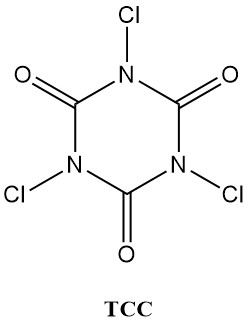
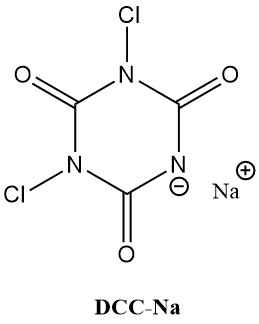
| Property | TCC | DCC-Na |
|---|---|---|
| CAS number | [87-90-1] | [2893-78-9] |
| Physical form | White powder | White powder |
| Formula | (ClNCO)3 | Cl2Na(NCO)3 |
| Mr | 232.44 | 219.98 |
| mp | 234 °C (decomp.) | 225 °C (decomp.) |
| Available chlorine Theoretical (%) | 91.5 | 64.5 |
| Available chlorine Typical value | 90.0 | 62.0 |
| pH (1 % aqueous solution) | 2.7 – 3.3 | 6.2 – 6.8 |
| Solubility in Water (25 °C) (g/100 g) | 1.0 | 30.0 |
| Solubility in Acetone (30 °C) | 35.0 | 0.5 |
TCC and DCC-Na have taken a significant market share historically dominated by calcium hypochlorite, pine oils, quaternary ammonium salts, and phenols.
N-chloroisocyanuric acids are produced by a continuous chlorination reaction of isocyanuric acid in aqueous sodium hydroxide at low temperatures (0–15 °C). Precise control over both pH and reaction temperature is important to prevent the formation of the highly explosive trichloroamine (NCl3).
Trichloroisocyanuric acid (TCC) decomposes gradually in an alkaline medium to generate byproducts like trichloroamine (NCl3), dichloroamine (NHCl2), and monochloroamine (NH2Cl).
Both sodium dichloroisocyanurate (DCC-Na) and its dihydrate have higher water solubility compared to TCC. DCC-Na dihydrate is obtained by cooling a saturated aqueous solution of DCC-Na (45°C) to 10°C; this form offers enhanced thermal stability compared to its anhydrous form.
The disinfection and sanitation power of N-chloroisocyanuric acids is due to their gradual release of hypochlorous acid (HOCl) in water, which exhibits both oxidizing and biocidal properties.
These compounds are used for many purposes, including:
- Nonshrinking treatment of wool: They safeguard wool fibers from shrinkage during processing.
- Swimming pool disinfection: N-chloroisocyanuric acids effectively eliminate harmful microorganisms in swimming pools, maintaining water hygiene.
- Cleaning and sanitizing bathrooms.
- Laundry bleach: they are used as effective laundry bleach to remove stains and enhance whiteness.
N-chloroisocyanuric acid-based cleaners and sanitizers often include additional components like phosphates, sodium metasilicates, surfactants, and neutral salts (sodium sulfate, sodium carbonate).
This combination enhances their oil and protein removal capabilities, making them ideal for various cleaning tasks. They are recommended for dishwashing in hotels, hospitals, restaurants, and food processing facilities due to their efficacy against various microorganisms.
5. Organic N-Chloroamines
Various organic N-chloroamine compounds and their applications are listed below:
1,3-Dichloro-5,5-dimethylhydantoin (Dactin) (4)
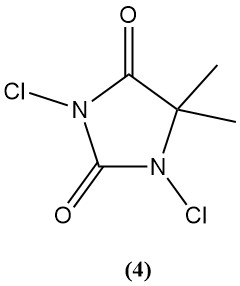
- Molecular weight: 197.03 g/mol
- Melting point: 132°C
- Theoretical chlorine content: 77.6%
- Solubility in water: 2.1 g/L
- It is prepared by chlorinating aqueous 5,5-dimethylhydantoin.
- It was used historically as a bactericide and disinfectant, but its use declined due to competition from TCC and Di-Halo.
1-Bromo-3-chloro-5,5-dimethylhydantoin (Di-Halo) (5)
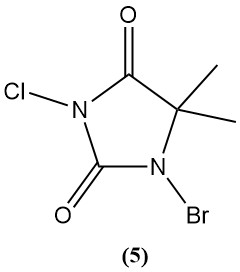
- Molecular weight: 241.48 g/mol
- Melting point: 130°C (decomp.)
- pH: 2.88 (0.1% solution)
- Water solubility (20°C): 2 g/L
- It is prepared by sequential bromination and chlorination of 5,5-dimethylhydantoin.
- It is a stable white powder with no chloroamine formation in weakly basic solutions.
- It is widely used as a swimming pool disinfectant (0.5–3 mg/L active halogen) due to its stability, broad-spectrum activity, and long shelf life.
1-Chloro-2,5-pyrrolidinedione (N-chlorosuccinimide) (6)
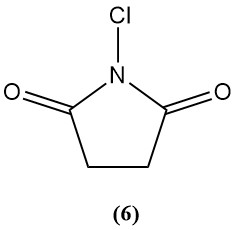
- Molecular weight: 133.54 g/mol
- Melting point: 150–151 °C
- It is sparingly soluble in various organic solvents.
- Available chlorine: 50–54%
- It is primarily used as an organic synthesis chlorinating agent.
N-Chloroglycolurils
- They are studied as protective agents against poison gas.
- They are prepared by 1,2-diketone condensation with urea, followed by chlorination.
- 2,4,6,8-tetrachloro-2,4,6,8-tetrazabi-cyclo[3.3.0]octane-3,7-dione (7) is an important example of this class.
![2,4,6,8-tetrachloro-2,4,6,8-tetrazabi-cyclo[3.3.0]octane-3,7-dione structure](https://chemcess.com/wp-content/uploads/2024/02/2468-tetrachloro-2468-tetrazabi-cyclo3.3.0octane-37-dione-structure.jpg)
1,3,4,6-Tetrachloro-3α,6α-diphenylglycoluril (Iodogen) (8)
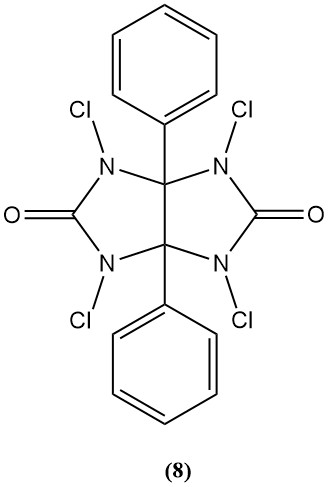
- Molecular weight: 431.94 g/mol
- It is used as a bactericide and oxidizing agent in peptide synthesis.
3-Chloro-4,4-dimethyl-2-oxazolidinon (9)
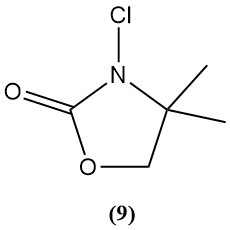
- Molecular weight: 149.50 g/mol
- It is an example of a new class of N-chloroamines under investigation as disinfectants.
Sodium N-chloro-p-toluenesulfonamide (Chloramine-T) (10)
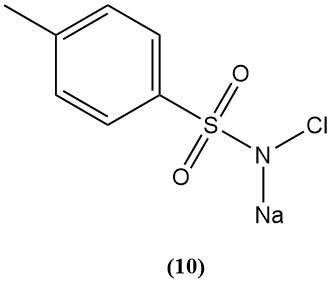
- Molecular weight: 227.67 g/mol (hydrate)
- Melting point: 175 °C; explosive
- Available chlorine: 25%
- It is prepared by chlorinating p-toluenesulfonamide in sodium hydroxide.
- It is a strong electrolyte in acid, a good oxidizing agent in base, and fairly soluble in water and insoluble in benzene.
- It reacts with mustard gas to form harmless sulfimide crystals.
- Their derivatives are studied for poison gas protection.
N,N’,N”-Trichloromelamine (11)
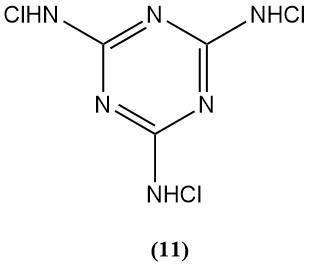
- Molecular weight: 229.46 g/mol
- Melting point: 175°C (decomp.),
- Theoretical chlorine content : 92.8%
- It is used as a sterilizing agent, limited by its low water solubility.
N-chlorinated amino acid derivatives are under investigation for their potential as bleaching agents and bactericides.
6. Toxicology of N-chloroamines
N-chloroamines are widely employed in drinking water, swimming pools, and food processing, which necessitate careful consideration of their potential toxicological effects.
When used in organic-rich water, particular vigilance is required to prevent the formation of weakly carcinogenic halomethanes. Their presence is often quantified through headspace, purge trap, or solvent extraction methods, followed by GC analysis with electron capture detectors, or GC-MS.
Most N-chloroamines exhibit local irritant effects on the eyes, moist skin, and the upper respiratory tract. Diluted solutions (up to 100 ppm available chlorine) of N-chloroisocyanuric acids typically do not pose toxicity, irritation, or sensitization concerns.
However, ingesting pure solids or concentrated suspensions can damage the stomach lining. Furthermore, isocyanuric acid, formed upon hydrolysis, possesses low inherent toxicity. A summary of acute toxicity values for select N-chloroamines is provided in Table 3.
| Compound | LD50 (mg/kg, rat, oral) |
|---|---|
| TCC | 1300 |
| DCC-Na | 1420 |
| 1,3-Dichloro-5,5-dimethylhydantoin | 542 |
| N-Chlorosuccinimide | 2700 |
- Chloramine-T: While a skin irritant, this compound presents significant toxicity upon absorption into the bloodstream, necessitating its use only in diluted solutions.
- Monochloroamine: Despite posing a lower halomethane formation risk, its widespread use in drinking water disinfection raises concerns due to potential mutagenic and aquatic toxicity. The U.S. Environmental Protection Agency has even proposed a prohibition on its use in drinking water.
Studies suggest that 1-bromo-3-chloro-5,5-dimethylhydantoin exhibits lower toxicity towards specific fish species compared to 1,3-dichloro-5,5-dimethylhydantoin.
Reference
- Chloroamines; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_553
