Cellulose Acetate Fibers: Properties, Production and Uses
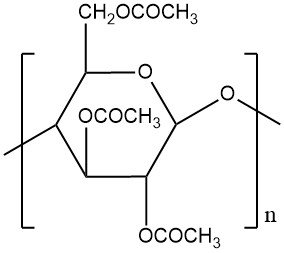
Cellulose acetate fibers are a type of synthetic fiber derived from cellulose. They are one of the earliest synthetic fibers ever developed and were once widely used in various applications. They are made by treating cellulose with acetic acid and acetic anhydride to create cellulose acetate, which is then dissolved in a solvent and spun into fibers through a dry spinning process.
Table of Contents
1. Properties of Cellulose Acetate Fiber
The spinning solution (dope) viscosity and filterability are important for cellulose acetate fiber production. Dope viscosity correlates with polymerization degree, influencing fiber strength, stretch, and acetate group distribution along the cellulose chain.
Small spinneret holes need good filtration of insoluble particles, mainly incompletely acetylated cellulose fibers or gels, which can clog the spinnerets.
Secondary and triacetate fibers share similar physical properties (Table 1), with densities lower than those of viscose rayon fiber and comparable to wool. Textile yarns require fibers with minimal color.
| Property | Secondary Acetate | Triacetate |
|---|---|---|
| Strength (cN/dtex) | 1.0 – 1.5 | 1.0 – 1.5 |
| Stretch (%) | 25 – 30 | 25 – 30 |
| Density (g/cm³) | 1.33 | 1.30 |
| Moisture Uptake (%) (65% relative humidity, 20°C) |
6 – 6.5 | 4 – 4.5 |
| Water Retention Capability (%) | 25 – 28 | 16 – 17 |
| Melting Point (°C) | 225 – 250 | Decomposition at 310 – 315 |
| DP | 300 | 300 |
Chemical reactions resemble those of organic esters. Strong acids and alkalis hydrolyze cellulose acetate, which is sensitive to strong oxidizing agents; however, hypochlorite and peroxide solutions have no impact.
Dyeing differences arise due to disparities in swelling properties. Cellulose acetate fibers require water-disperse dyes at boiling temperatures, typically with carrier assistance. Carriers facilitate fiber swelling and enhance dye uptake.
This integrated process ensures colorfastness. Triacetate fibers exhibit superior wash-and-wear properties due to their enhanced dimensional stability and wrinkle resistance.
2. Raw Materials
Wood pulp is the primary cellulose source for acetate fiber production. Softwood (conifer) or hardwood (deciduous) species can be used, processed through either the sulfite pulping method with hot alkali extraction or the prehydrolyzed sulfate (Kraft) process with cold caustic extraction.
Both methods aim to remove lignins and hemicelluloses, resulting in purified wood pulps containing over 96% α-cellulose. Economic factors have rendered high-purity cotton linters obsolete in this domain.
High-quality acetate fibers need wood pulp with specific characteristics. Good swelling properties are crucial for ensuring even exposure of cellulose to the catalyst and acetylation agent during processing. In addition, the pulp should yield a spinnable solution without fibers and gels for easy filtration.
3. Production of Cellulose Acetate Fibers
The production follows the general principles outlined in the article about cellulose acetate, with key differences. Initially, sulfuric acid catalyzes the formation of cellulose sulfateester, which is, later replaced by acetyl groups during acetylation.
Hydrolysis further reduces the remaining sulfate groups. However, any residual sulfate must be neutralized with stabilizers like magnesium salts to prevent heat- and humidity-induced degradation via sulfuric acid release.
Secondary acetate uses acetone as a solvent, while triacetate employs 90% dichloromethane and 10% methanol/acetic acid (wet-spinning). The spinning solution’s viscosity, with 20–30% cellulose acetate, ranges from 300 to 500 Pa·s at 45–55 °C. Filtration and deaeration are important steps before spinning.
Dry spinning dominates, with wet spinning occasionally used for triacetate. Spinnerets possess 20–100 holes for filament and up to 1000 for tow. Solvent evaporation occurs in a 4-6 m spinning column at 80–100 °C using a countercurrent air flow.
The fibers are stretched to enhance strength while in a plastic state. Melt spinning lacks commercial viability due to its limited heat stability.
Triacetate fibers exhibit a core-skin structure due to the highly regular distribution of acetyl groups. Heat setting (crystallization) at 180–200 °C improves wash-and-wear properties.
This process requires several minutes at 180 °C or a few seconds at 220 °C to optimize water retention (10%) and absorption (2.5%). Shorter durations are ineffective, and longer periods deteriorate the textile’s mechanical properties.
4. Uses of Cellulose Acetate Fibers
Combining cellulose acetate or triacetate fibers with nylon or polyester offers a synergistic blend of properties ideal for various lining applications. This compensates for the weaker physical characteristics of acetate fibers while retaining their desirable qualities, such as high moisture absorption and silk-like softness.
Due to their unique blend of hydrophobic and hydrophilic properties, semipermeable membranes made from cellulose acetate fibers are used for water desalination by reverse osmosis.
Cellulose acetate hollow fibers also find use in gas separation and hemodialysis applications.
Cellulose acetate’s non-toxic nature, biodegradability, and reliance on renewable natural polymers (cellulose) open the possibility for promising future applications in various fields.
Reference
- Cellulose Esters; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a05_419.pub2
