Carbazole: Properties, Production and Uses

Carbazole is a heterocyclic aromatic hydrocarbon with the chemical formula C12H9N. It was first isolated from coal tar in 1872 by Graebe and Glaser. Its structure features a dibenzopyrrole consisting of a five-membered nitrogen-containing ring fused to two benzene rings.
Table of Contents
1. Physical Properties of Carbazole
Carbazole is a white to pale yellow crystalline solid with a distinct odor. Its key physical properties include:
- Molar mass: 167.21 g/mol
- Melting point: 246 °C
- Boiling point: 354.8 °C at 101.3 kPa
- Density: 1.1035 g/cm³ at 18 °C
- Crystal morphology: plates or tables; sublimable
- Solubility:
- Highly soluble: acetone, pyridine
- Moderately soluble: diethyl ether, ethanol
- Slightly soluble: chloroform, acetic acid, carbon tetrachloride, and carbon disulfide
- Very soluble: concentrated sulfuric acid
- Practically insoluble: water (≈ 1 mg/L at 25 °C)
- Thermodynamic properties:
- Heat of fusion: 176.3 kJ/kg
- Heat of combustion: 3.719 × 10⁴ kJ/kg at 25 °C
2. Chemical Reactivity of Carbazole
Carbazole exhibits diverse reactivity due to its aromatic nature and the lone pair on its nitrogen atom. Here’s a breakdown of its principal chemical reactions:
1. N-Substitution and Alkylation
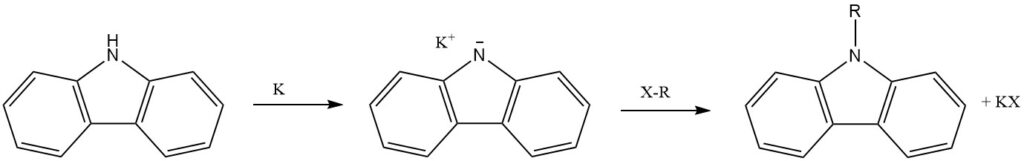
The N-hydrogen of carbazole, acting as a secondary amine, readily undergoes substitution with alkali metals (Li, Na, and K). This leads to the introduction of other functional groups through further reactions.
The N-atom of carbazole can be alkylated, introducing N-alkyl substituents with varying properties.
2. Halogenation, Nitration, and Sulfonation

Electrophilic substitution reactions like halogenation, nitration, and sulfonation preferentially target the 3- and 6-positions on the carbazole ring due to their higher electron density. Harsh conditions are necessary for further substitution at the 1-, 8-, and other positions, leading to 1,3,6- and 1,3,6,8-derivatives.
It can also undergoes alkylation and acylation reactions on the aromatic ring.
3. Hydrogenation
Carbazole readily undergoes hydrogenation, yielding partially or fully saturated derivatives. Depending on the reaction conditions, 1,2,3,4-tetrahydro-, hexahydro-, or even dodecahydrocarbazole can be formed.
4. Oxidation
Oxidation with strong oxidizing agents like chromates or permanganates cleaves the aromatic ring, forming different dicarbazyl products depending on the cleavage site. 3,3′-dicarbazyl is formed by chromate oxidation, while 9,9′-dicarbazyl is obtained from oxidation with permanganate.
5. Carboxylation

Treatment with alkali metal carbonates and carbon dioxide can introduce carboxylic acid groups onto the carbazole ring. The position of the carboxyl group (3- or 1-) depends on the reaction temperature.
3. Production of Carbazole
Key Points:
- Carbazole is readily available in coal tar and can be separated from anthracene using various methods.
- Synthetic routes exist but are currently less competitive than coal tar extraction.
- Modern high-temperature melt crystallization offers a promising approach for sustainable carbazole production
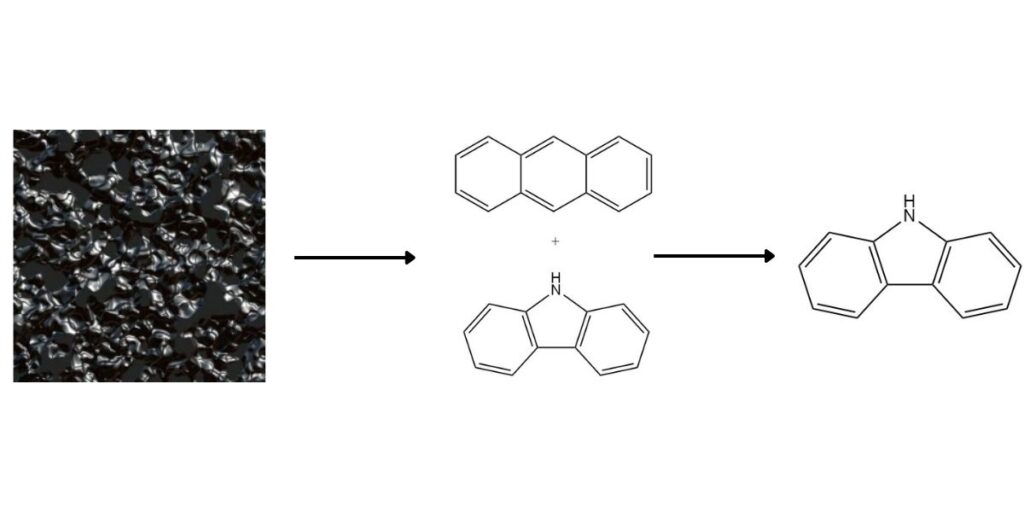
Carbazole as a valuable heterocyclic aromatic compound, is present in high-temperature coal tar at an average concentration of 0.9%. While traditionally obtained as a co-product during anthracene production, several methods offer dedicated carbazole recovery.
1. Separation from Anthracene:
Carbazole’s higher boiling point and differential solubility facilitate its separation from anthracene. Common methods include:
- Extraction or complexation: Utilizing solvents like pyridine, ketones, benzene/methanol mixtures, or specific agents like N-methylpyrrolidone, dimethylacetamide, dialkyl sulfoxides, or dialkylformamides.
- Azeotropic distillation: Employing ethylene glycol to exploit the formation of an azeotrope with carbazole, enabling separation by fractional distillation.
- Selective hydrogenation: Converting anthracene to hydrogenated derivatives while leaving carbazole unaffected.
2. Chemical Separation:
Historically, methods like potassium hydroxide or concentrated sulfuric acid fusion were used for carbazole separation. However, their economic viability has diminished.
3. Purification from Pyridine Mother Liquors:
Pyridine mother liquors generated during anthracene purification can be concentrated and recrystallized from chlorobenzene to yield pure carbazole.
4. Synthetic Routes for Carbazole
Several synthetic methods exist for carbazole production, but their industrial significance is limited compared to coal tar extraction:
- Conversion of cyclohexanone azine to octahydrocarbazole followed by dehydrogenation.
- Reductive cyclization of 2-nitrobiphenyl.
- Dehydrogenation and cyclization of various precursors like diphenylamine, o-aminobiphenyl, or N-cyclohexylideneaniline.
A modern and efficient method for carbazole recovery from coal tar is solvent-free, high-temperature melt crystallization. This process offers advantages in terms of environmental impact and cost-effectiveness.
4. Uses of Carbazole
Despite being primarily extracted from coal tar (over 3,500 tonnes annually), carbazole is used in various industries including: dyes and pigments, pesticides and polymers, optical materials and light-emitting diodes and in construction and adhesives.
Dyes and Pigments:
- Hydron Blue: the condensation of carbazole with p-nitrosophenol and subsequent sulfurization yields this commercially important blue sulfur dye.
- Naphtol AS and Anthraquinone Dyes: Carbazole serves as a precursor for various azo and anthraquinone dyes used in textile and industrial applications.
- Styryl Dyes: N-ethylcarbazole enables the synthesis of vibrant styryl dyes used in diverse materials and products.
- Dioxazine Dyes and Pigments: N-ethyl-3-aminocarbazole plays a key role in creating these valuable colorants for organic pigments and azine dyes.
- Carbazole contributes to the production of the reactive dyes, known for their strong bonding to synthetic fibers.
Pesticides and Polymers:
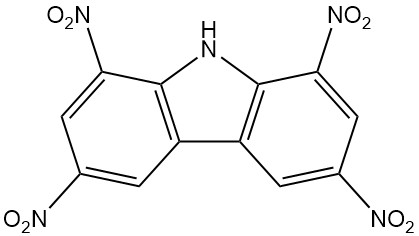
- Nirosan: 1,3,6,8-tetranitrocarbazole, an effective insecticide, has been commercially available since 1939.
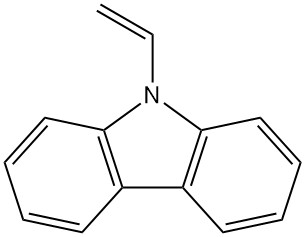
- Poly(vinylcarbazole): N-vinylcarbazole polymerization yields this thermally and chemically stable polymer (Luvican, Polectron) used for electrical applications due to its low dielectric loss and photoconductivity.
Optical Materials and Light-Emitting Diodes:
- Photorefractive Materials: Methacrylate carbazole polymers combine photoconductivity and electro-optic properties, making them ideal for high-density data storage, image processing, and computing.
- Triplet Emitters: Carbazole compounds find use as host materials for triplet emitters in organic light-emitting diodes (OLEDs), enhancing their efficiency and brightness.
Construction and Adhesives:
- Novolak Resins: Carbazole reacts with phenols and formaldehyde to form these heat-resistant polymers, valuable for adhesives and coatings.
- Concrete Plasticizers: Cocondensation of carbazole with phenols and formaldehyde, followed by sulfonation, yields effective plasticizers for concrete.
High-performance Polymers:
- Polyamides: Thermal condensation of 3,6-diaminocarbazole with dicarboxylic acids produces elastic and thermally stable polyamides with diverse applications.
5. Carbazole Derivatives

| Derivative | CAS Number | Formula | Molar Mass (g/mol) | Melting Point (°C) | Synthesis |
|---|---|---|---|---|---|
| N-Ethylcarbazole | 86-28-2 | C14H13N | 195.27 | 68 | Ethylation of carbazole with diethyl sulfate |
| 1,3,6,8-Tetranitrocarbazole | 4543-33-3 | C12H5O8N5 | 347.20 | 312 | Nitration of carbazole with nitric and sulfuric acid |
| N-Vinylcarbazole | 1484-13-5 | C14H11N | 193.25 | 65 | From carbazole-potassium with ethylene oxide or vinyl chloride, or from carbazole and acetylene |
6. Toxicology of Carbazole
Carbazole exhibits low acute oral toxicity, with LD50 values exceeding 5000 mg/kg in rats. However, intraperitoneal administration in mice reveals significantly lower LD50 of 200 mg/kg, suggesting route-dependent toxicity.
Occupational exposure to carbazole has been associated with folliculitis and comedos, though animal studies demonstrate minimal skin and eye irritation potential, even under photoactivation.
Carbazole undergoes glucuronidation and subsequent urinary excretion in rats and rabbits.
High-dose carbazole feeding (270-1050 mg/kg bw/day) for 96 weeks induced liver and forestomach carcinomas in B6C3F1 mice, but no tumorigenic activity was observed in newborn mice after intraperitoneal injections.
Based on limited evidence, carbazole is classified as Group 3 (not classifiable as to carcinogenicity to humans) by the International Agency for Research on Cancer (IARC). It had no mutagenic activity in various bacterial assays.
9-Ethylcarbazole, a derivative, showed a weak mutagenic effect in one bacterial strain but was inactive in another, suggesting limited mutagenic potential.
Dermal application of carbazole at up to 250 mg/kg bw/day during pregnancy did not induce abnormalities in rat fetal development.
References
- Carbazole; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a05_059.pub2
- Carbazole; – https://www.sigmaaldrich.com/US/en/product/sigma/c5132
- https://en.wikipedia.org/wiki/Carbazole
