
Neutral dyes are those that do not change the pH of the solution in which they are dissolved. They are often used to dye synthetic fibers, such as polyester and nylon, which are not affected by acidic or alkaline dyes.
Four types of neutral dyes are : disperse dyes, vat dyes, pigments, and dyes soluble in organic solvents.
Table of Contents
1. Disperse Dyes
Disperse dyes are water-insoluble dyes that are used to color synthetic fibers, such as polyester, polyamide, and acetate. They are especially useful for dyeing polyester fibers.
Disperse dyes are typically anthraquinone compounds with hydroxy or amino groups as auxochromes. These compounds can be used to create a wide range of colors, from bright red to blue. Other dye classes are used for yellow and orange shades.
The choice of substituents on the dye molecule can be tailored to specific fibers and desired colors.
1.1. Dyes for Polyester Fibers
Anthraquinone dyes are used to color polyester fibers. There are five main types of anthraquinone dyes, which are chosen based on their affinity for the fiber, sublimation resistance, and lightfastness.
Larger substituents in the side chains tend to increase sublimation resistance, but decrease dye affinity. The position and hydrophilic nature of substituents also affect affinity. In some cases, mixtures of dyes or contaminants can improve affinity through synergistic effects.
Negatively charged substituents, such as carboxylic esters, halogen, or sulfone groups, can improve lightfastness.
1-Amino-4-hydroxyanthraquinones are known for their good lightfastness and affinity for polyester fibers, particularly in creating bright red dyes. The brilliance of these dyes can be further enhanced by introducing ether groups ortho to the amino groups.
Aliphatic ethers surpass aromatic ethers in terms of lightfastness and exhibit a somewhat brighter yet more yellow hue. Additional substituents in the side chains can enhance sublimation resistance.
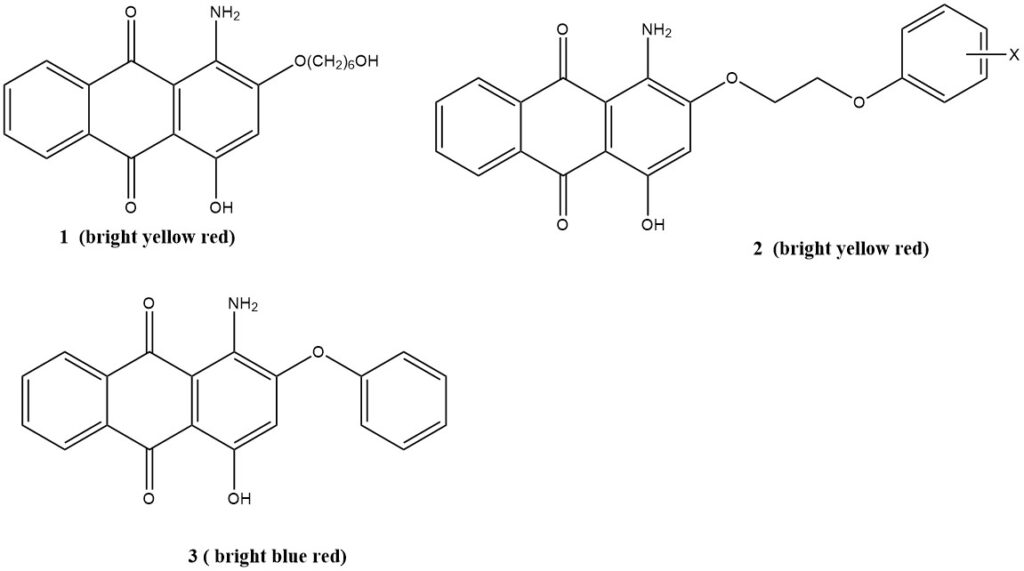
The typically poor lightfastness of 1,4-Diaminoanthraquinones can be ameliorated by the incorporation of appropriate negatively charged substituents. For example, the 2-sulfophenyl ester is a sublimation-resistant, brilliant blue dye with a reddish undertone and commendable lightfastness.
The nitration into the 2 position shifts the dye shade toward bluish green while enhancing resistance to sublimation and fading.
The addition of chlorine atoms in the β-position significantly improves lightfastness with minimal impact on basic sublimation characteristics.
Furthermore, β-Phenoxy groups in the 2,3 positions can shift the shade to a bright, somewhat reddish violet, offering good stability against fading and sublimation.

N-Substituted 4-Amino-1-hydroxyanthraquinones, which are alkyl- or aryl- aminohydroxyanthraquinones, are recognized for their affinity and lightfastness, resulting in violet to blue shades.
Nevertheless, they often fall short in terms of sublimation fastness when compared to their tetrasubstituted counterparts. Substituents like carboxylic esters, arylsulfonic esters, amides, hydroxyethylether, and methoxy groups in arylamino compounds can enhance sublimation resistance. Optimal blends can prevent a reduction in affinity.

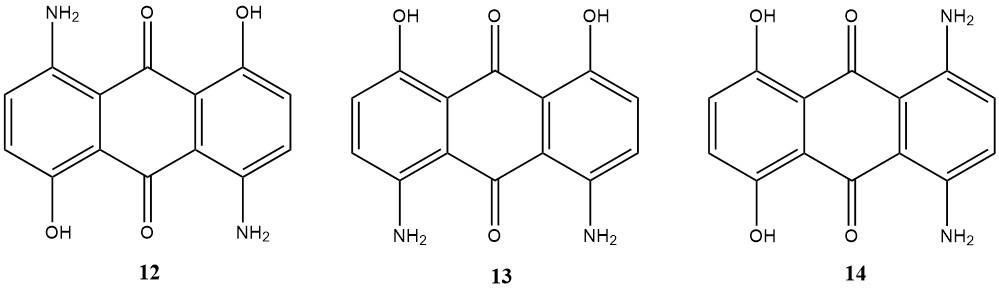
Finally, diaminodihydroxyanthraquinones, derived from α-diaminodihydroxyanthraquinones, are essential disperse dyes due to their shade and affinity versatility. Tailoring dye properties can be achieved by strategic substituent introduction, isomer position selection, and blending.
The bathochromic shift affects color, with lightfastness improving in the order of 13, 14, 12, and affinity increasing from 12, 14, 13. Sublimation fastness, however, remains moderate.
Halo, alkoxy, hydroxyaryl, and phenylmercapto derivatives have gained commercial importance, with substitution adjacent to the amino group leading to brighter dyes and enhanced affinity.
For instance, 15 is a well-known example of C.I. Disperse Blue 56, and 16 is C.I. Disperse Blue 73.

Nitroarylaminodihydroxyanthraquinones, recognized for their greenish-blue shades and superior light and sublimation fastness, undergo improvements in affinity upon reduction.
Example: 17 represents a dye of this kind, characterized by its blue hue.

1.2. Dyes for Cellulose Ester and Synthetic Polyamide Fibers
Disperse dyes were first developed to dye cellulose fibers, but their importance has declined since the development of synthetic fibers. Synthetic polyamide fibers can be dyed with dyes that were originally developed for cellulose acetate fibers, and very few new dyes have had to be developed specifically for polyamide fibers.
The types of dyes used for polyamide fibers are similar to those used for polyester fibers, but they are chosen based on different criteria. While sublimation resistance is not as important for polyamide fibers, fastness to ozone, exhaust gases, and washing is important.
Unlike for polyester fibers, substituting amino groups, especially alkylamino groups, into disperse dyes used for polyamide fibers does not reduce lightfastness.
The orange derivatives of 1-aminoanthraquinone are not very important for dyeing polyamide fibers because they have a relatively low tinctorial strength. In contrast, the derivatives of 1-amino-4-hydroxyanthraquinone are highly valuable, yielding brilliant red dyes.
The most important dyes in this category are derived from 1,4-diaminoanthraquinone, which offer shades ranging from violet to greenish blue. Blending similar compounds can significantly enhance affinity.
Examples of dyes in this category include:
- Dye 18: A brilliant red.
- Dye 19: C.I. Disperse Blue 14, used in Celliton Fast Blue from BASF.
- Dye 20: A brilliant blue.
- Dye 21: C.I. Disperse Blue 31, used in Celliton Blue 3 G from BASF.
- Dye 22: C.I. Disperse Blue 7, used in Celliton Blue Green B from BASF.

1.3. Transfer Dyes
Transfer printing is a method of printing on polyester fabrics using disperse dyes. The dye is coated onto transfer paper, and then the fabric is pressed against the paper at a high temperature. The heat causes the dye to sublimate, or turn into a gas, and then diffuse into the fibers of the fabric.
Dyes specially formulated for transfer printing have been developed, but readily available disperse dyes can also be used. Examples of transfer dyes include:
- Dye 23 (C.I. Disperse Red 60, [17418-58-5])
- Dye 24 (C.I. Disperse Blue 26, [3860-63-7])

1.4. Dyes for Cotton – Polyester Fabrics
Anthraquinone dyes with a moderate molecular weight can be used to directly print and dye cellulose fibers, such as cotton-polyester fabrics that have been pretreated with water. Most of these dyes are disperse dyes with excellent sublimation resistance. However, low-molecular-weight vat dyes are also included in this group.
These dyes are typically applied with high-boiling, water-miscible solvents such as glycols and glycol derivatives, or boric acid esters of compounds with one to six hydroxy groups. The fabrics are first swollen with water, and then heat-treated at around 200°C to evaporate the water and allow the dye to enter the fiber through its solution phase. The polyester component of the fabric is also dyed during this process.
Examples of such dyes include:
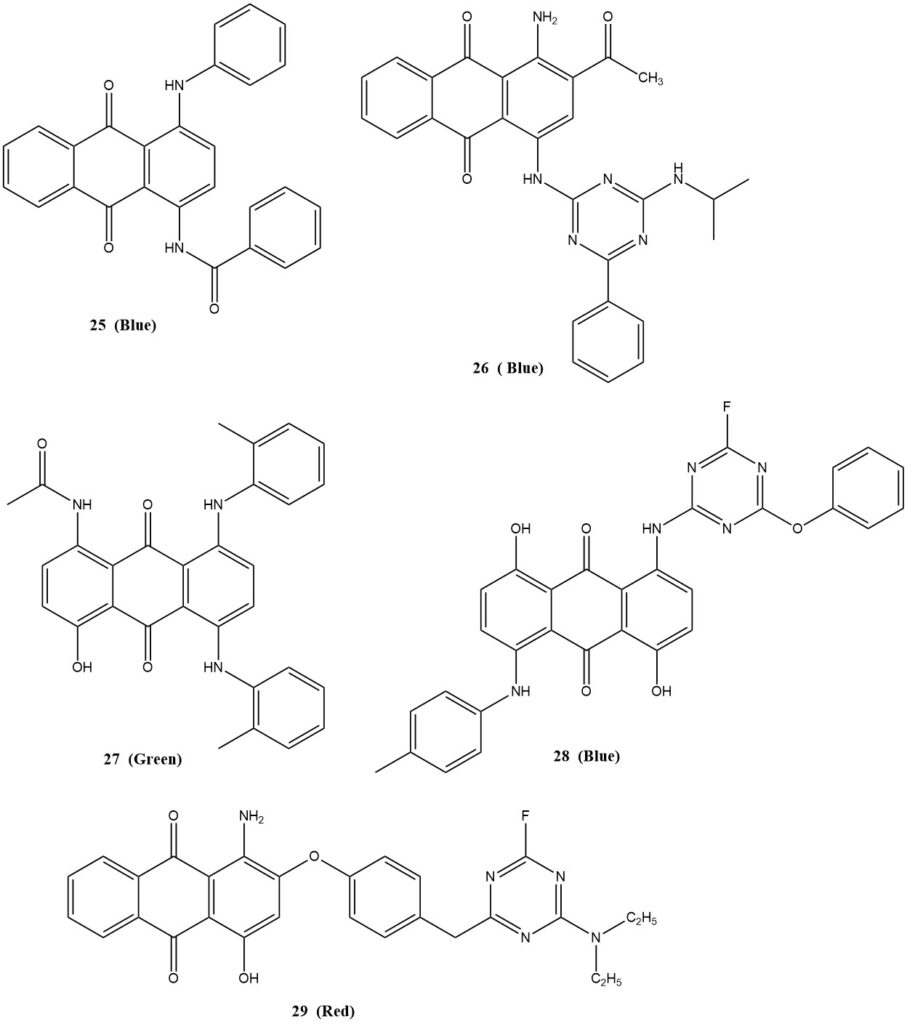
- Compound 25 (blue dye)
- Compound 26 (blue dye)
- Compound 27 (green dye)
Reactive disperse dyes can also be used to achieve uniform dyeing on cotton-polyester fabrics. Fluorotriazine reactive dyes have been proposed for this specific application.
Examples of these dyes include:
- Compound 28 (blue dye)
- Compound 29 (red dye)
2. Dyes Soluble in Organic Solvents
Anthraquinone dyes with simple structures are used to color substances such as gasoline, oil, and plastics. For example, bis(alkylamino)anthraquinones are highly soluble and can be used to color gasoline, while quinizarin and its derivatives are used to mark heating oils. The addition of alkali can change the color of these dyes.
Examples of such dyes include:
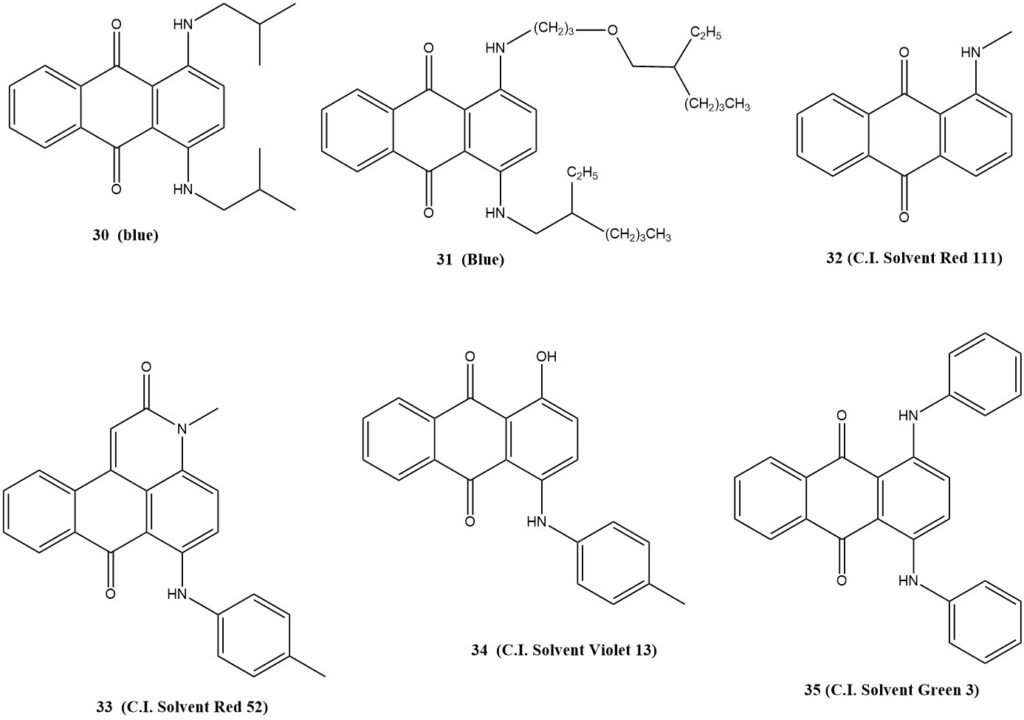
- Compound 30: A blue dye used to color hydrocarbons, including gasoline.
- Compound 31: A mixture of blue dyes used to color gasoline and mineral oils.
To dye amorphous thermoplastics such as polymethacrylate, polystyrene, or polycarbonate, dyes that can dissolve in the material and maintain transparency are required. These dyes can also be used in combination with substances such as titanium dioxide to give thermoplastics body colors.
Initially, compounds with relatively simple structures were used, often derived from existing intermediates. However, newer products designed to meet specific requirements have since become available.
Examples of such dyes include:
- Compound 32 (C.I. Solvent Red 111, [82-38-2])
- Compound 33 (C.I. Solvent Red 52, [81-39-0])
- Compound 34 (C.I. Solvent Violet 13, [81-48-1])
- Compound 35 (C.I. Solvent Green 3, [128-80-3])
Many relatively simple amino- and hydroxyanthraquinone derivatives exhibit a high degree of order in liquid crystalline systems. Therefore, they are suitable as dichroic dyes for guest-host displays.
3. Vat Dyes
Vat dyes are a class of water-insoluble dyes that are used to color cotton and other cellulose fibers. They are known for their excellent colorfastness, but they can be expensive and have a limited color range.
Vat dyes are converted into soluble leuco compounds by reducing agents in the presence of sodium hydroxide. These leuco compounds are then able to penetrate cellulose fibers. After reoxidation, the insoluble dye strongly bonds with the fiber.
Acylaminoanthraquinones are the simplest type of vat dye. They are produced by acylating aminoanthraquinones, typically with benzoic acid or benzoyl chloride.
These dyes offer good affinity for cellulose fibers, but simple types like 1,4- (and 1,5-) dibenzoylaminoanthraquinones are no longer as significant.
Bridging linkages, such as oxalic or phthalic acids, can be used to couple two anthraquinone units, resulting in a variety of colors.

Anthraquinoneazoles exhibit good light fastness and are derived from 1-aminoanthraquinone-2-carboxylic acid and 3-amino-2-hydroxy- (or -mercapto-) anthraquinones. The blue derivatives of 1-amino-4-aroylaminoanthraquinone-2-carboxylic acid are especially noteworthy for their resistance to atmospheric conditions and chlorine.
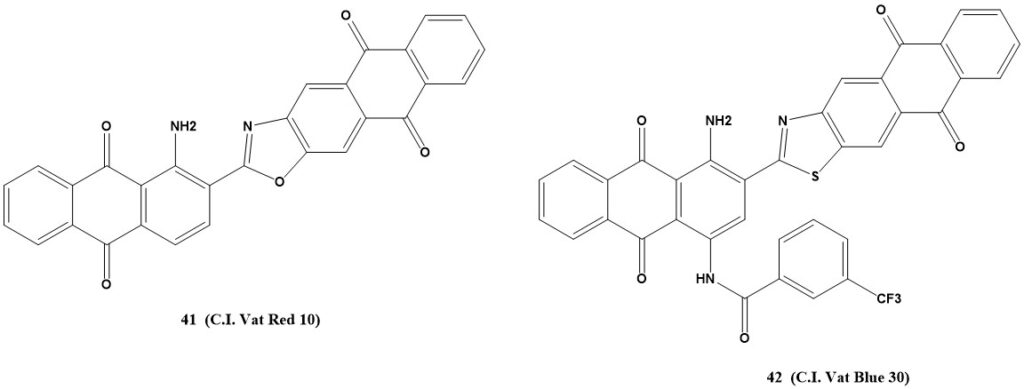
Anthrimides and other linked anthraquinones primarily feature α,β derivatives among anthrimides as significant vat dyes. Compounds that link two anthraquinone molecules through functional derivatives of the 2-aldehyde (or 2-carboxy) group are also utilized. These dyes offer good colorfastness.

Anthrimide carbazoles are produced by carbazole ring closure from α,α-dianthraquinonylamines (anthrimides). These dyes offer level, very fast colors like yellow, orange, brown, gray, and olive for cellulose fibers.
However, bright shades are somewhat lacking in this series.
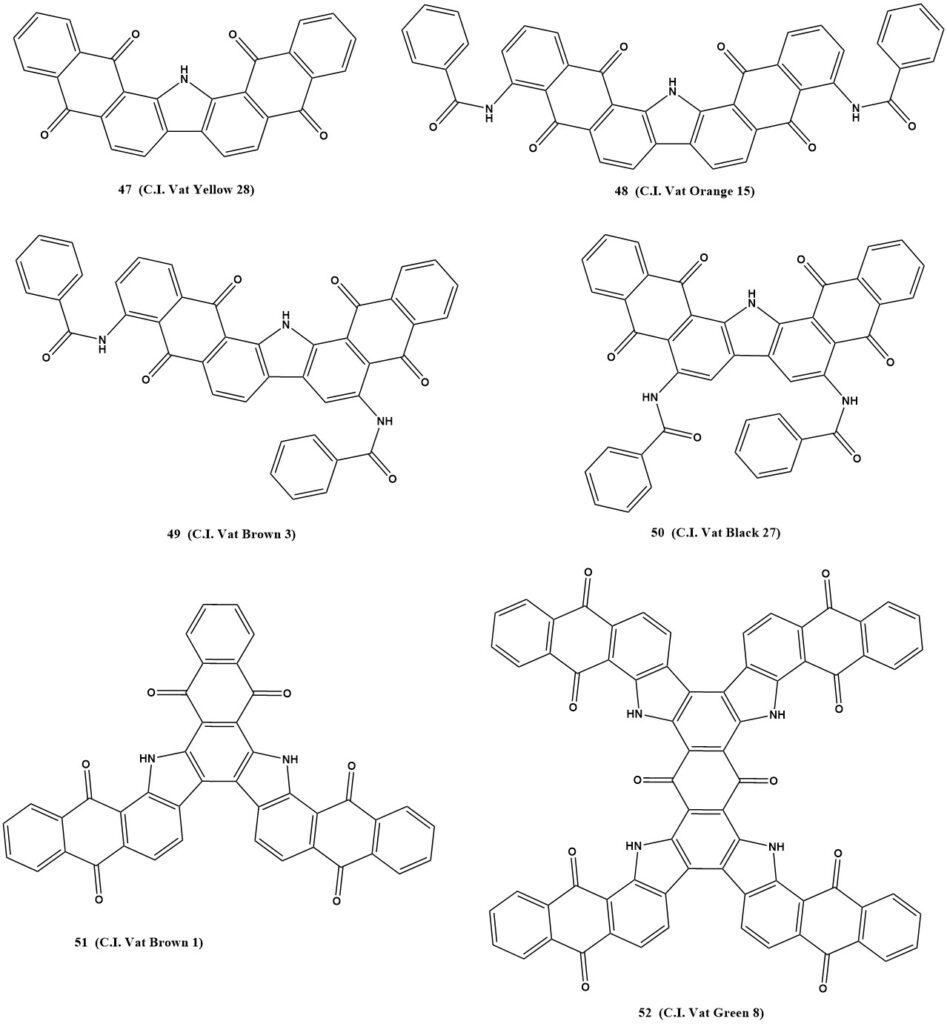
Phthaloylacridones provide shades ranging from red to green, depending on the substituent in the 2 position. They offer good light fastness but somewhat poorer wash fastness.

Benzanthrone dyes can be divided into two main groups: “imide-green” dyes and dyes from the violanthrone and isoviolanthrone series. Imide-green dyes exhibit muted colors like olive green, olive, khaki, and grey, along with excellent resistance to light and atmospheric conditions. The blue color of violanthrone can be transformed into a brilliant green with specific modifications.
Indanthrones comprise the blue indanthrone, one of the first synthetic vat dyes in the anthraquinone series. Despite its low resistance to chlorine, it remains essential due to excellent colorfastness and vivid colors. Hydroxy or amino group introductions can shift the shade towards green.
Highly condensed ring systems provide valuable additions to anthraquinone vat dyes. These include dibenzpyrenequinone, anthanthrone, and pyranthrone, which offer yellow to red vat dyes without extra auxochromic substituents. Halogenation can enhance their affinity and alter their shades. Some of these derivatives can be further converted to anthrimide-like compounds.
Vat dyes are an important class of dyes that are used in a variety of applications. They are known for their excellent colorfastness and durability, but they can be expensive and have a limited color range.
4. Anthraquinone Pigments
Many vat dyes can also be used as pigments due to their limited solubility in organic substances. However, they must be carefully prepared to achieve a high degree of purity and specific physical characteristics, such as crystal structure and particle size distribution.
Anthraquinone pigment production can be expensive, so they are only used in specialized applications where exceptional colorfastness is crucial. Only a few vat dyes meet these strict requirements.
Most pigments belong to the category of dyes with polycondensed ring systems, such as indanthrones, anthanthrones, pyranthrones, flavanthrone, and their halogenated derivatives, apart from certain acylaminoanthraquinones.
Over the past 25 years, a range of specialized anthraquinone dyes have been developed as pigments, including anthraquinone-azo, bianthraquinonyl, and anthraquinonylaminotriazine derivatives.
There is currently limited interest in colored lakes, especially aluminum lakes of hydroxyanthraquinones such as alizarine, purpurin, quinizarin, and their sulfonic acids.
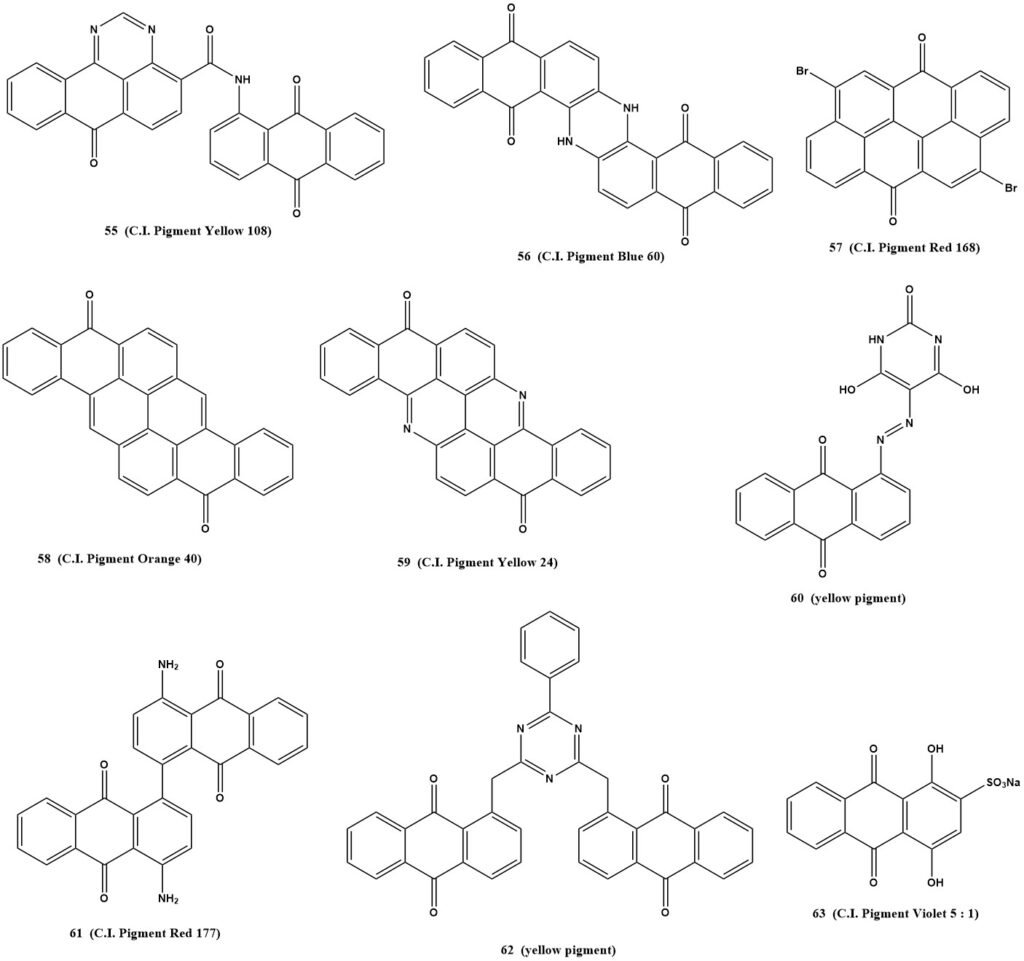
Examples of such pigments include:
- C.I. Pigment Yellow 108 (55)
- C.I. Pigment Blue 60 (56)
- C.I. Pigment Red 168 (57)
- C.I. Pigment Orange 40 (58)
- C.I. Pigment Yellow 24 (59)
- Yellow pigment (60)
- C.I. Pigment Red 177 (61)
- Yellow pigment (62)
- Aluminum Lake, C.I. Pigment Violet 5:1 (63)
Reference
- Anthraquinone Dyes and Intermediates; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_355


