Amino Resins
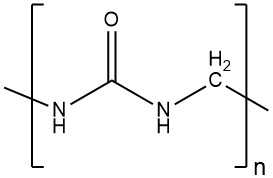
Amino resins are condensation polymers formed by the reaction of carbonyl compounds with compounds containing amino, imino, or amide groups. The reaction releases water, and the resulting products are oligomers, also known as prepolymers.
A wide variety of carbonyl- and nitrogen-containing compounds can be used to produce amino resins, but the most common are formaldehyde, urea, and melamine. These compounds are used because they are relatively inexpensive and easy to obtain. The type of chemical reaction that occurs determines the molecular weight of the prepolymer.
Table of Contents
Amino resins are produced on a large scale in modern industrial plants. They offer a number of technical advantages, including good adhesion, water resistance, and chemical resistance.
The history of amino resins began with the synthesis of urea and formaldehyde in the 19th century. The first commercial amino resins were produced in the early 20th century. A major breakthrough occurred in the 1930s with the development of resin-bonded particle board.
Today, amino resins are used in a wide variety of applications, including:
- Adhesives for particle board, MDF, plywood, and other wood products
- Laminated beams and parquet flooring
- Furniture assembly
- Paper strengthening
- Molding compounds
- Carpet backing
- Leather auxiliaries
- Soil conditioners
- Protective surface coatings
- Decorative paper saturation
Amino resins are a versatile and important class of polymers with a wide range of applications.
2. Physical Properties of Amino Resins
Amino resins are available commercially in two forms: concentrated solutions and solids, such as powders.
The solutions are typically water-based, but alcohols are used as solvents in surface-coating resins. The solutions range in color from colorless and clear to milky and cloudy. They are also tacky to the touch and have a high viscosity, typically 20–70,000 mPa·s (measured at 20 °C).
The odor of amino resin solutions can vary depending on the free formaldehyde content, ranging from odorless to characteristic to sharp. The density of the solutions at the commercially available concentrations of 50–80% solids is 1.15–1.31 g/cm3.
An important property of amino resin solutions is their water tolerance, or the amount of water that can be added without precipitating solids. At relatively low temperatures, below about 20°C, many amino resin solutions exhibit pronounced cloudiness and an unusually large increase in viscosity.
Some amino resin solutions also exhibit the phenomenon of pseudoplasticity (thixotropy), which means that the viscosity decreases as the shearing force increases. Thixotropy is not always desirable, but it can be useful in some applications.
In powder form, amino resins are white. The commercially available spray-dried powders have a particle size of 15–70 μm, but always less than 200 μm. The bulk density of the powders is 0.5–0.8 kg/L.
When amino resin powders are dissolved in water, they typically form milky liquids. However, in some cases, clear solutions can be achieved. Flour-like additives can be mixed with some amino resins, which can then assume the color of the additive to a greater or lesser extent.
3. Chemical Reactions of Amino Resins
Amino resins are mixtures of various substances, even when they are prepared from only two components. For example, urea–formaldehyde resins are composed of hydroxymethyl compounds, oligomers, and methylene–urea. The chemical properties of amino resins are common to all or most of them.
One of the important chemical properties of amino resins is that formaldehyde is slowly split off from hydroxymethyl compounds and undergoes addition reactions with other amino groups.
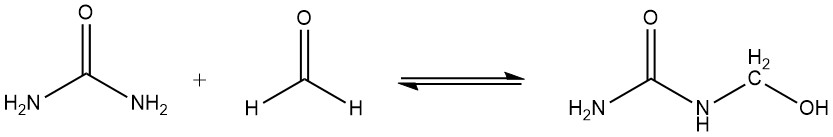
Another important chemical property of amino resins is that hydroxymethyl compounds undergo slow condensation in the absence of heat or react with urea to give methylene compounds. This reaction is responsible for the gradual increase in viscosity that amino resins undergo during storage.

These reactions are accelerated by acid catalysts and elevated temperatures. Hence, acids or acid-forming substances are used as hardeners that accelerate the condensation in processing amino resins.
Amino resin solutions are generally rendered slightly alkaline, i.e., pH 7.5–9, in order to stabilize them. However, the pH slowly decreases as formic acid is formed. This effect is more pronounced for urea–formaldehyde resins and much less significant in melamine–formaldehyde resins.
The hydroxymethyl groups in amino resins can be converted with alcohols into the corresponding ethers. This reaction is utilized in surface-coating resins and impregnation resins for the manufacture of paper-based edge-banding material.
The free formaldehyde, which is present in concentrations from 0.03% to about 2%, is of practical importance. It reacts with ammonium salts of acids to give hexamethylenetetramine and the free acid, which acts as a hardener.
Resin powders also condense over time to give relatively high molecular mass products, but in this case the reaction is slower by a factor of at least five.
4. Raw Materials for Amino Resins
The raw materials for amino resins are formaldehyde, urea, melamine, thiourea, and substituted ureas. Guanamines and aniline, are also employed.
- Formaldehyde is the most important raw material for amino resins. It is available in the form of solid paraformaldehyde, aqueous solution, or urea-formaldehyde precondensate.
- Urea is the second most important raw material for amino resins. It is available as a white crystalline product or as prills.
- Melamine is a less common raw material for amino resins. It is a white powder that sublimes on heating.
- Thiourea is a relatively expensive raw material that is not used in large quantities.
- Substituted ureas are a group of urea derivatives that are used in specialized applications.
In addition to these raw materials, a variety of modifiers can be used to improve the properties of amino resins. These modifiers include aliphatic alcohols, glycols, sugars, sugar alcohols, polysaccharides, and caprolactam.
The choice of raw materials and modifiers for amino resins depends on the specific application. For example, urea-formaldehyde resins are commonly used in adhesives, while melamine-formaldehyde resins are used in laminates and dinnerware.
Reference
- Amino Resins; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_115.pub2
