3-Methyl-2-butanone: production and uses

3-Methyl-2-butanone is a clear liquid with a characteristic ketone scent.
Condensation reactions occur preferentially at the methyl group of 3-Methyl-2-butanone.
Oxidation of the tertiary carbon atom occurs easily and produces hydroperoxide (melting point 26-27 °C) via base-catalyzed air oxidation.
The use of condensation reactions of the methyl group and reactions of the carbonyl group for synthesis have been reported.
Table of Contents
1. Production of 3-Methyl-2-butanone
The industrial production of 3-Methyl-2-butanone involves the following reactions:
- A single-step condensation of 2-butanone, formaldehyde, and hydrogen over palladium or a two-step condensation on a strongly acidic cation exchanger, followed by isolation of 2-methyl-1-buten-3-one and hydrogenation.

2. Gas-phase reaction of isobutyraldehyde with acetaldehyde over manganese oxide – aluminum oxide at 450 °C.

Isobutyric acid and acetic acid can be used in place of isobutyraldehyde and acetaldehyde.
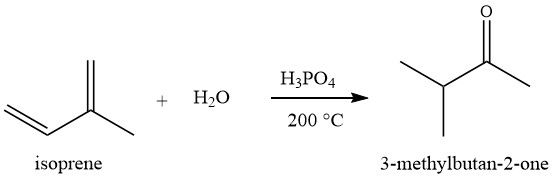
3. By reacting Isoprene with water at around 200 °C in the presence of phosphoric acid on silica.
4. Oxidation of 2-methyl-2-butene with oxygen or hydroperoxides in the presence of molybdenum naphthenate at 150 °C.

2. Uses of 3-Methyl-2-butanone
3-Methyl-2-butanone is primarily used as an intermediate for the production of pharmaceuticals, herbicides, and dye precursors.
It is also used in the synthesis of rubber auxiliaries and for the selective extraction of rare earth elements.
Reference
- Ketones; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a15_077
