Sorbic Acid: Properties, Reactions, Production and Uses
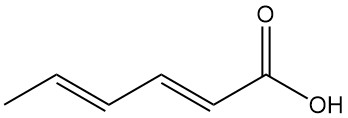
What is Sorbic Acid?
The trans, trans isomer of 2,4-hexadienoic acid, commonly known as sorbic acid, is a short-chain unsaturated fatty acid with the chemical formula C6H8O2. It is a naturally occurring organic acid that appears as colorless solid with a faint odor.
Sorbic acid derives its name from “Sorbus aucuparia Linnaeus,” the scientific nomenclature of the mountain ash. In 1859, Hofmann isolated an oil with a distinctive odor from unripe rowan berries by distillation.
The primary constituent of this oil was parasorbic acid, identified as the d-lactone of sorbic acid. Strong acids, or alkalis, catalyze the conversion of parasorbic acid to isomeric sorbic acid. Unripe rowan berries contain approximately 0.1% parasorbic acid.
Sorbic acid is found in the fatty deposits of certain aphids as 2-sorboyl-1,3-dimyristin. Doebner established sorbic acid’s structure in 1890, and it was first synthesized in 1900.
Müller (Germany) and Gooding (United States) independently discovered sorbic acid’s antimicrobial properties in 1939–1940. Industrial production of sorbic acid and potassium sorbate started in the 1950s, initially in the United States, followed by Germany and Japan.
Sorbic acid is used as a primary food preservative because of its safety and favorable sensory characteristics.
Table of Contents
1. Physical Properties of Sorbic Acid
Sorbic acid forms needle- or plate-like crystals with a mild, characteristic odor and a slightly acidic taste. Its solubility in water is limited, with values of 0.16 g, 0.58 g, and 3.9 g per 100 mL at 20 °C, 50 °C, and 100 °C, respectively.
The solubility of sorbic acid in anhydrous lower-molecular weight alcohols and anhydrous acetic acid is approximately 11–12 g per 100 mL. Liquid fats can dissolve 0.5–1 g of sorbic acid per 100 mL.
Sorbic acid is volatile in steam without decomposition. This property is used for its analytical isolation from food products.
The important physical properties of sorbic acid are listed in the following table.
| Property | Value |
|---|---|
| CAS number | [110-44-1] |
| Formula | C6H8O2 |
| Molecular mass | 112.13 g/mol |
| Melting point | 132–135 °C |
| Boiling point | 228 °C (decomposition) |
| Sublimation temperature | above 60 °C |
| Refractive index | 1.4248 |
| Density | 1.204 g/cm3 |
| Dissociation constant | 1.73×10-5 at 25 °C |
| pKa | 4.76 |
| Vapor pressure at 20 °C | <0.001 kPa |
| Vapor pressure at 100 °C | 0.25 kPa |
| Vapor pressure at 120 °C | 1.3 kPa |
2. Chemical Reactions of Sorbic Acid
Sorbic acid reactivity is characterized by the presence of a carboxyl group and a conjugated double bond system.
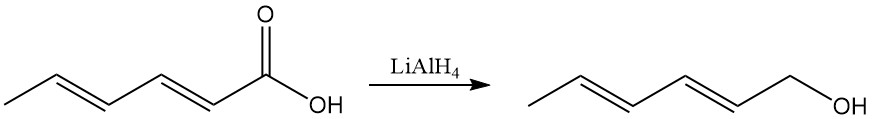
Carboxyl acid reactions include typical reactions like the formation of salts, esters, and other derivatives. Lithium aluminum hydride selectively reduces the carboxyl group to sorbyl alcohol (2,4-hexadien-1-ol).
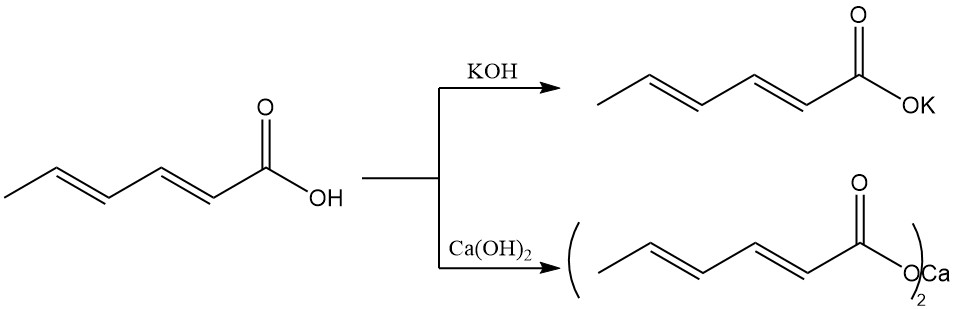
Unlike sorbic acid, alkali salts have higher water solubility, making them preferred for aqueous preservation systems. Potassium sorbate is produced from sorbic acid and potassium hydroxide and has good solubility in water.
Calcium sorbate is formed from sorbic acid and calcium hydroxide and is characterized by low water solubility.
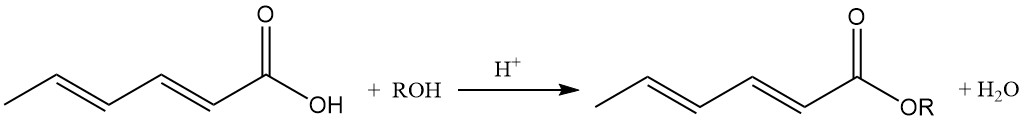
Lower alkyl sorbate esters possess antimicrobial properties and are active in neutral and weakly alkaline environments, unlike sorbic acid.
Reactions involving the double bond system often yield complex product mixtures due to varying double bond reactivities and potential isomerizations, rearrangements, migrations, and polymerizations.
Addition reactions and partial hydrogenation preferentially target the 4,5 double bond.
Chlorine addition results in chlorohexenoic acid mixtures with an average chlorine content of 38–48 wt%. The reaction of bromine with sorbic acid in organic solvents produces 2,3,4,5-tetrabromohexanoic acid, while aqueous bromination primarily yields 4,5-dibromo-2-hexenoic acid.
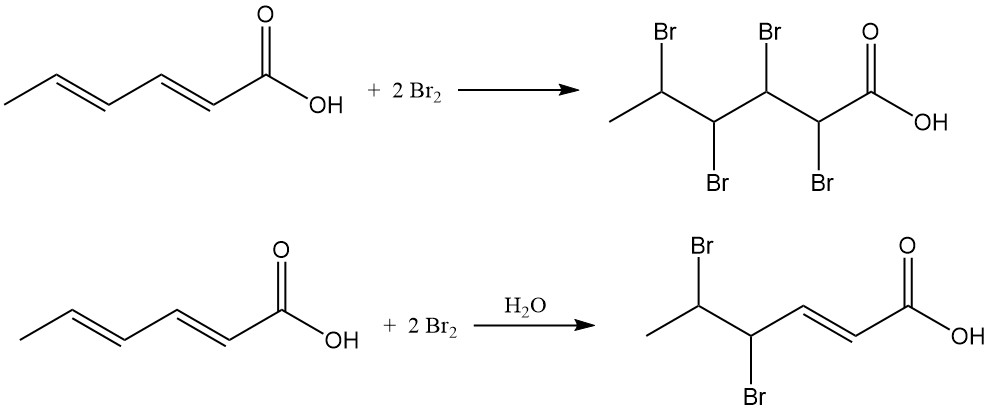
Despite its double unsaturation, pure crystalline sorbic acid shows high resistance to air oxidation, remaining stable for years under room-temperature conditions. However, impurities like solvents, heavy metals, or isomeric hexadienoic acids significantly reduce its stability.
Similar considerations apply to potassium sorbate, while sodium sorbate is unstable in solid form and is not commercially produced. Sodium sorbate rapidly converts to the sodium salt of 4,5-epoxy-2-hexenoic acid in the presence of air.
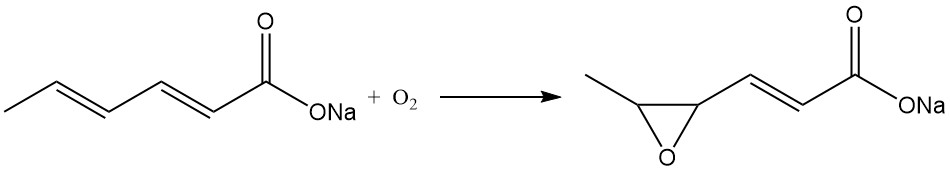
Oxidation of aqueous sorbates or sorbic acid in organic solvents generates numerous carbonyl compounds, although this reaction depends upon the presence of a large quantity of oxygen and is strongly increased by exposure to sunlight.
Oxygen-free solutions maintain stability even under light exposure. Sorbic acid in intermediate-moisture foods undergoes gradual degradation over months in the presence of air.
The oxidation of acidic aqueous sorbic acid solutions with potassium dichromate produces malonaldehyde. The reaction of malonaldehyde with 2-thiobarbituric acid produces a red, fluorescent 1:2 (malonaldehyde:2-thiobarbituric acid) adduct, used as a color indicator in sorbic acid analysis.
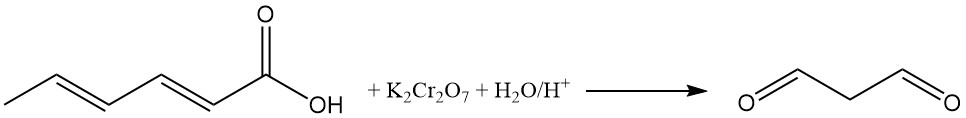
When incorporated into food, sorbic acid stability generally matches that of essential food components like vitamins, flavors, and aromas. Therefore, the theoretical autoxidation potential of sorbic acid poses minimal practical preservation challenges.
3. Production of Sorbic Acid
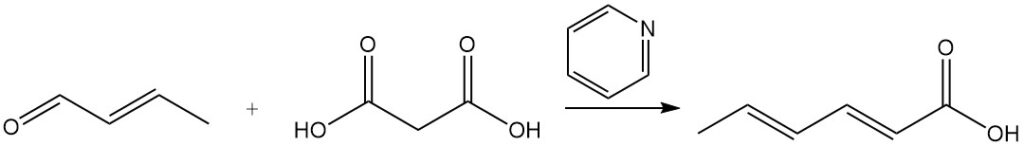
Sorbic acid was initially synthesized by the Doebner process, which involved a condensation reaction between crotonaldehyde and malonic acid in pyridine. While many other syntheses use similar principles, most are commercially impractical due to low yields, expensive starting materials, or high production costs.
This also applies to a 1950s–1960s Union Carbide process that oxidized 2,4-hexadienal to sorbic acid with catalysts. The process was abandoned due to the formation of up to 20% isomeric hexadienoic acids, which are less stable than sorbic acid and require complex purification. Microorganisms can also oxidize 2,4-hexadienal to sorbic acid.
Alternatively, sorbic acid can be produced by isomerizing 2,5-hexadienoic acid (1) by boiling with aqueous alkali. 2,5-Hexadienoic acid is formed by the reaction of allyl chloride with acetylene, carbon monoxide, and water using a tetracarbonylnickel catalyst.
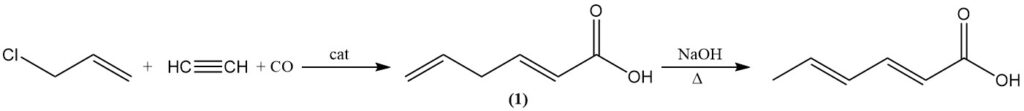
The acidic cleavage of 5-vinyl-γ-butyrolactone, which is obtained from 1,3-butadiene and acetic acid with redox catalysts, is not of industrial importance. Carbon dioxide addition to 1,3-pentadiene in the presence of nickel complexes is another possible synthesis.
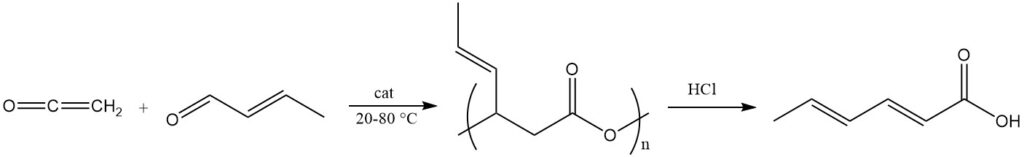
A common process uses ketene and crotonaldehyde in the presence of divalent transition metal salts as catalysts at a temperature of 20–80 °C to yield a polymeric ester of 3-hydroxy-4-hexenoic acid with an approximate molecular mass of 2000.
This polyester is cleaved to yield sorbic acid by using base or acid treatment (e.g., hydrochloric acid) or metal-complex catalysts. This polyester is also used as a precursor for the synthesis of sorbate esters by reactions with alcohols in the presence of acidic esterification catalysts.
Crude sorbic acid is purified by recrystallization from an aqueous solution, water-alcohol or acetone mixtures, or water-immiscible organic solvents like methyl acetate. Organic solvent treatment and steam distillation have also been reported.
4. Uses of Sorbic Acid
Sorbic acid and its salts (primarily potassium and calcium sorbates) are predominantly used as preservatives due to their low toxicity profile. It is active against molds and yeasts. The primary application areas include:
- Food: cheese (various types), baked goods, fruit products (prunes, pulps, marmalades, jams, juices, jellies, and candies), wine, fermented vegetables, margarine, butter, and sausages.
- Animal feed
- Tobacco
- Cosmetics
- Pharmaceuticals
- Packaging materials for food, animal feed, cosmetics, and pharmaceuticals
- Other products that come into contact with human or animal skin
It is worth noting that while potential uses for sorbic acid in chemical reactions and polymerizations have been explored in the patent literature, these applications remain commercially insignificant due to its relatively high cost.
Sorbic acid can also be used as an additive in cold rubber production and as an intermediate in the manufacturing process of certain plasticizers and lubricants.
5. Toxicology of Sorbic Acid
Sorbic acid exhibits low acute toxicity, with an LD50 of approximately 10 g/kg body weight in rats. Studies report similar values ranging from 7.4 to 8.7 g/kg. While sorbic acid can irritate mucous membranes due to its acidic nature, skin irritation is uncommon.
Extensive toxicological investigations have been conducted on sorbic acid due to its early introduction as a food preservative and mandatory testing requirements. Acute, subacute, subchronic, and chronic studies consistently demonstrate a high safety profile.
Sorbic acid has low allergenic potential due to its small molecular size, preventing antibody formation and covalent protein binding, which are typically associated with immediate hypersensitivity. Instances of pseudo-allergic reactions to sorbic acid as a food additive are rare.
Subchronic studies in rats show increased growth and liver weight at high sorbic acid doses (10% in feed), which was interpreted as hypertrophy due to increased metabolic workload rather than toxicity.
Long-term feeding studies (two years) in rats and mice demonstrate no carcinogenic effects at low sorbic acid concentrations (1.5–10% in feed). Higher doses (10%) lead to reduced weight gain and an enlarged thyroid, liver, and kidneys.
Sorbic acid is neither teratogenic nor mutagenic or genotoxic, and it is non-carcinogenic.
Metabolically, sorbic acid undergoes β-oxidation similar to other fatty acids.
Both sorbic acid and potassium sorbate show low allergenic and phototoxic potential. A single case of type 4 allergic reaction has been reported despite widespread use for over 40 years. The Cosmetic Ingredient Review (CIR) Expert Panel classifies both substances as “safe” for cosmetics.
Sodium sorbate, not commonly used in foods due to oxidation susceptibility, shows low genetic toxicity in vitro, unlike sorbic acid and potassium sorbate.
References
- Sorbic Acid; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a24_507.pub2
- https://pubmed.ncbi.nlm.nih.gov/2079232/
