
Vitamin B3, also known as niacin, belongs to the B complex vitamins. Nicotinic acid and nicotinamide are the two major forms of vitamin B3, and the terms niacin or vitamin B3 are often used interchangeably to describe either of these two compounds.
Pyridine 3-carboxylic acid and vitamin PP are other names for nicotinic acid, while pyridine 3-carboxylic acid amide, pyridine 3-carboxamide, vitamin PP, and niacinamide are commonly used to refer to nicotinamide.
Nicotinic acid was synthesized for the first time in 1867 via oxidative degradation of nicotine, well before its role as an essential nutrient for preventing pellagra was discovered.
In the early 1900s, it was found that pellagra was caused by a deficiency in nicotinic acid.
Naturally occurring niacin exists in two forms, nicotinic acid in plant-based foods, and nicotinamide in animal-based products. However, not all of the natural niacin present in foods is bioavailable to humans and animals.
Cereals and oil seeds contain niacin in a bound complex, and the average niacin content in food products ranges from 10 to 200 mg/kg.
Table of Contents
1. Production of Vitamin B3
Industrial production of nicotinic acid and nicotinamide is based on the raw materials 5-ethyl-2-methylpyridine and 3-methylpyridine (3-picoline).
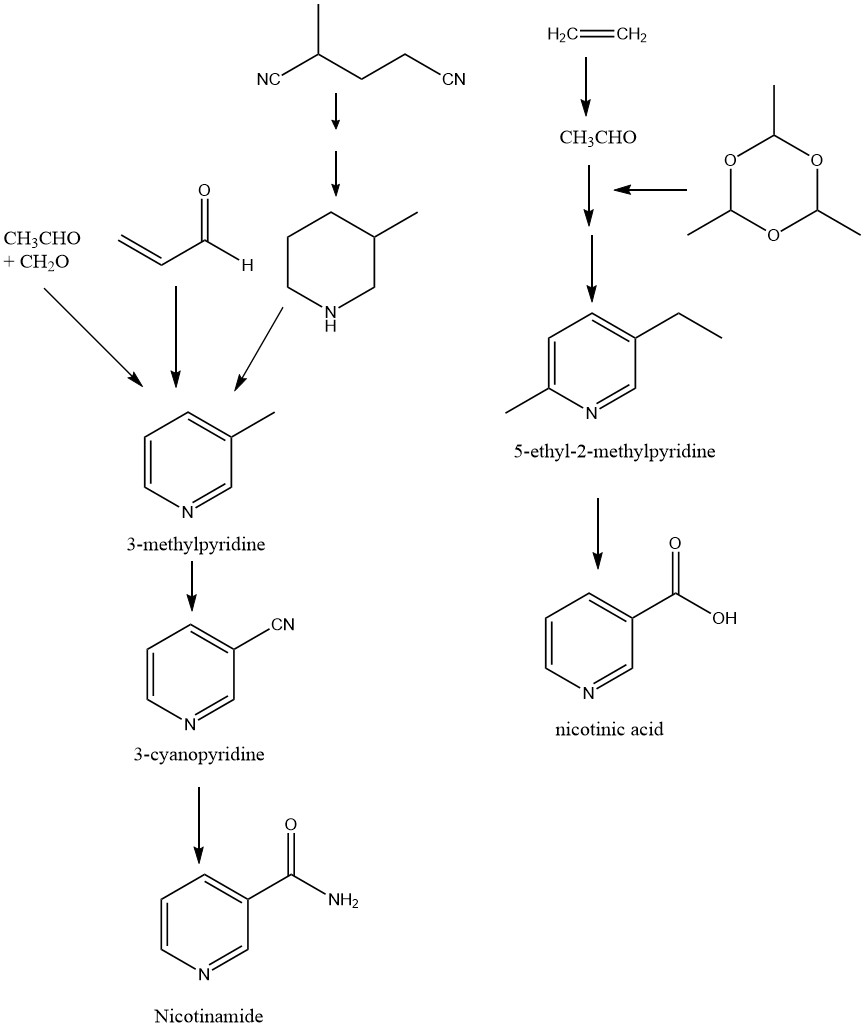
3-Methylpyridine is a byproduct obtained by catalytic gas-phase reaction of acetaldehyde, formaldehyde, and ammonia. However, the main product is pyridine. The yield of 3-methylpyridine is approximately 30-50%. 5-Ethyl-2-methylpyridine, on the other hand, is produced by the reaction of paraldehyde with ammonia.
Nicotinic acid is obtained directly via nitric acid oxidation of 5-ethyl-2-methylpyridine or through the complete hydrolysis of 3-cyanopyridine.
Nicotinamide, on the other hand, can be obtained by the amidation of nicotinic acid or by partial hydrolysis of 3-cyanopyridine. The latter is obtained through ammoxidation of 3-methylpyridine.
1.1. Production of Nicotinic Acid by Oxidation of 5-Ethyl-2-methylpyridine
The process of oxidation of 5-Ethyl-2-methylpyridine is commonly used to produce nicotinic acid. The world’s largest manufacturing plant for nicotinic acid is situated in Visp, Switzerland, and is operated by Lonza.

In an aqueous solution, nicotinic acid is produced from 5-ethyl-2-methylpyridine in a one-step process utilizing excess nitric acid within a continuous reactor at 230–270 °C and 6–8 MPa.
During this process, pyridine-2,5-dicarboxylic acid is generated as an intermediate but is unstable under the reaction conditions and subsequently undergoes decarboxylation to nicotinic acid nitrate.
The nitrate is then neutralized with 5-ethyl-2-methylpyridine to yield nicotinic acid.
Carbon dioxide and water formed during the reaction are separated from the product, while the nitrogen oxide produced is oxidized with air to nitrogen dioxide and subsequently absorbed in water to obtain nitric acid, which is reused in the process.
1.2. Production of Nicotinamide by Ammoxidation of 3-Methylpyridine and Hydrolysis of 3-Cyanopyridine
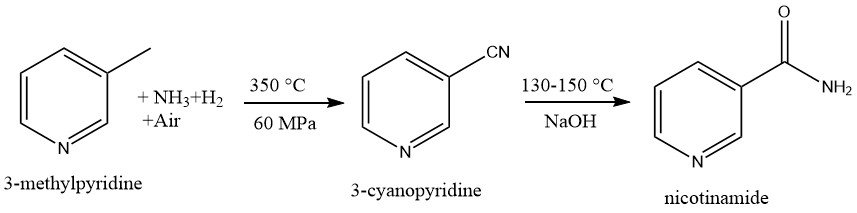
In a multi-tubular reactor, 3-methylpyridine undergoes a reaction with air, ammonia, and hydrogen at approximately 350°C and moderate pressure to produces 3-cyanopyridine.
Heterogeneous catalysts, which contain oxides of antimony, vanadium, and titanium, antimony, vanadium, and uranium, or antimony-vanadium-titanium catalyst, exhibit high efficiency.
For example, with a vanadium, titanium, zirconium, molybdenum catalyst, a reactor temperature of 340°C, and a molar feed ratio of 3-methylpyridine: ammonia: oxygen of 1:1.3:40, 95% of 3-cyanopyridine is obtained.
Nicotinamide is produced by the alkaline hydrolysis of 3-cyanopyridine. This reaction has the advantage that saponification to the amide is faster than total hydrolysis to nicotinic acid.
The hydrolysis to the amide usually involves catalytic amounts of bases, mainly sodium hydroxide, at 130–150°C.
In the Lonza process, 3-cyanopyridine is converted to nicotinamide using an immobilized microorganism of the genus Rhodococcus. Heterogeneous catalysts are also mentioned.
An alternative method is hydrolysis of 3-cyanopyridine to nicotinamide in aqueous fluids under high pressure.
1.3. Production of Nicotinamide by Conversion of 2-Methylglutaronitrile
2-Methylglutaronitrile, which is produced as a byproduct during adiponitrile production, can be transformed into 2-methyl-1,5-diaminopentane. Subsequently, the process of cyclic hydrogenation can be employed to convert this intermediate into 3-methylpiperidine.
Upon dehydrogenation, 3-methylpiperidine is transformed into 3-methylpyridine. The latter is then subjected to ammoxidation and partial hydrolysis to obtain nicotinamide.

2. Biochemical Functions of Vitamin B3
Nicotinamide is an essential component of the diet primarily as NAD and NADP, which are hydrolyzed in the intestine, resulting in the release of nicotinamide. Nicotinamide can be absorbed as it is, or after being hydrolyzed to nicotinic acid.
In cereals, nicotinic acid exists as a glycoside that undergoes only partial hydrolysis in vivo. As a result, its absorption from the gastrointestinal tract is limited, and it is therefore poorly bioavailable.
Nicotinic acid is rapidly absorbed from both the stomach and the upper small intestine. Niacin is also biosynthesized via kynurenine and quinolinic acid as key intermediates.
Niacin acts as a precursor for two crucial coenzymes, nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). These coenzymes play a critical role in the transfer of metabolic hydrogen, a fundamental process in the metabolism of fats, carbohydrates, and proteins.
This function is necessary for both the synthesis and degradation of amino acids, fatty acids, and carbohydrates. The niacin coenzymes also play a critical role in the citric acid cycle. The citric acid cycle includes several steps in which activated acetate is repeatedly oxidized.
Compounds from the degradation of amino acids, carbohydrates, and fatty acids are first broken down to pyruvate oxal-acetate, then enter the cycle with active acetate.
The various oxidative steps of the cycle produce a significant amount of energy that is stored in adenosine triphosphate (ATP).
This energy is released by converting ATP to adenosine diphosphate (ADP), which can then store more energy. Consequently, niacin plays a vital role in the metabolic production and utilization of energy.
3. Uses of Vitamin B3
Pellagra, a deficiency disease caused by insufficient intake of niacin, was previously widespread in areas where corn was the primary source of nutrition.
While subclinical symptoms of niacin deficiency still appear under conditions of malnutrition or one-sided nutrition, the enrichment of cereal products with B vitamins, particularly with nicotinic acid or nicotinamide, has become standard practice and required in some countries since the early 1940s.
Breakfast cereals, multivitamin drinks, and multi-vitamin tablets are examples of niacin-rich products.
The European Commission has approved and published claims regarding the beneficial effects of niacin, including its contributions to normal psychological function, energy-yielding metabolism, functioning of the nervous system, maintenance of normal mucous membranes and skin, and reduction of tiredness and fatigue.
Niacin is also essential for most animals, but biosynthesis from tryptophan is relatively insignificant, and natural feedstuffs are generally poor in this amino acid.
Nicotinic acid is synthesized by the microflora in the large intestine of monogastric animals and in the rumen of ruminants, but biosynthesis in monogastrics is insignificant due to its occurrence in the large intestine, which is located after the main niacin-absorption sites of the duodenum and small intestines.
Most of the nicotinic acid synthesized by the microflora is thus excreted in the feces.
Niacin is necessary for the optimal health and performance of all domestic animals and pets.
The biosynthesis of nicotinic acid in the rumen is suboptimal under high production stress or when the rumen microflora has been disturbed. Hence, niacin levels should be higher than the National Research Council (NRC) recommendations to meet the requirements of animals.
Pharmacological doses of nicotinic acid generate numerous pharmacological responses, such as effects on lipid metabolism, vasodilation, and activation of fibrinolysis. Interestingly, few if any of these activities are shared by nicotinamide, despite its close relationship with nicotinic acid.
For instance, plasma cholesterol and triglyceride concentrations significantly decrease with doses of 1–2 g/d of nicotinic acid, which is available in different products such as nicotinic acid or esters of nicotinic acid.
Niacin is also used in electroplating baths, particularly in the zinc electroplating process, where it is present in a quaternized form obtained by the reaction of niacin with benzyl chloride, resulting in uniform and brilliant surfaces.
Reference
- Vitamins, 8. Vitamin B3 (Niacin); Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.o27_o14.pub2


