Protocatechuic Acid: Properties, Production and Uses

3,4-Dihydroxybenzoic acid, also known as protocatechuic acid, is a naturally occurring organic acid with the formula C7H6O4. It is a white or slightly off-white crystalline solid that is soluble in some organic solvents like ethanol and ether, but insoluble in water.
3,4-Dihydroxybenzoic acid is found naturally in various plants, fruits, and vegetables, including:
- Salvia miltiorrhiza (known as Danshen in traditional Chinese medicine)
- Camellia sinensis (green tea leaves)
- Fruits like apples, plums, and grapes
Protocatechuic acid is currently being investigated as a food additive, pharmaceutical agent, cosmetic ingredient, and agricultural pesticide. It is generally considered safe, but high doses should be avoided.
Table of Contents
1. Physical Properties of 3,4-dihydroxybenzoic Acid
- Molecular formula: C7H6O4
- Molar mass: 154.1 g/mol
- Appearance: Light brown solid
- Melting point: 203 °C
- Density: 1.524 g/cm³ (at 4 °C)
- Melting Point: 202 °C (396 °F; 475 K)
- Solubility in Water: 18 g/L (at 14 °C), 271 g/L (at 80 °C)
- Solubility in alcohol and ether: soluble
2. Chemical Reactions of 3,4-Dihydroxybenzoic Acid
3,4-Dihydroxybenzoic acid (Protocatechuic acid) is a type of phenolic acid that can undergo various chemical reactions:
- Decarboxylation to produce catechol when heated with alkali.
- Eterification with methyl halogen or dimethyl sulfate to produce vanillic acid.
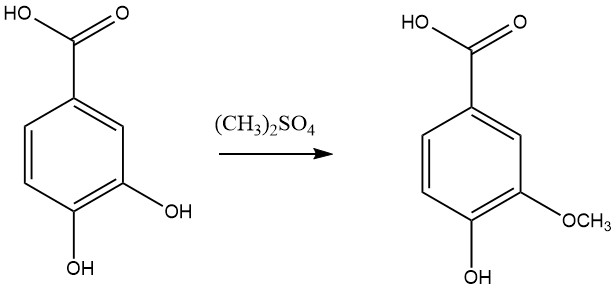
- Reduction to produce protocatechualdehyde and 4-(hydroxymethyl)benzene-1,2-diol.

- 3,4-Dihydroxybenzoic acid can form various esters, such as methyl 3,4-dihydroxybenzoate and ethyl 3,4-dihydroxybenzoate, by esterification with methanol and ethanol,respectively.
- Condensation with other compounds to produce polymers and other derivatives
- Protocatechuic acid can undergo other aromatic reactions such as: nitration, acylation and alkylation.
Biosynthesis
In biological systems, 3,4-dihydroxybenzoic acid is a product of the shikimate pathway, which is a seven-step metabolic route used by bacteria, fungi, algae, parasites, and plants for the biosynthesis of aromatic amino acids.
3. Production of Protocatechuic Acid
3.1. Production of Protocatechuic Acid from Vanillin

Alkaline fusion of vanillin is the most practical method for synthesizing protocatechuic acid. This reaction oxidizes vanillin to vanillic acid, with the release of hydrogen. Demethylation of vanillic acid to protocatechuic acid occurs to a limited rate at 210–235 °C, but complete demethylation only occurs at temperatures above 240–245 °C.
To synthesize protocatechuic acid from vanillin, sodium hydroxide, potassium hydroxide, and water are mixed in a stainless-steel reactor. Iron or nickel can also be used. The mixture is heated and stired until it reaches 160 °C.
Vanillin is added gradually, maintaining the temperature at 190–195 °C. Continueous stirring and heating is maintened until the temperature reaches 240–245 °C. After 5 minutes of reaction the heating is stopped and the mixture is stirring as it cools.
Once the mixture has cooled to 150–160 °C, water is added and stired until the fusion mixture dissolves completely. After passing sulfur dioxide gas through the mixture for 2 minutes and Acidify the mixture completely with hydrochloric acid, the acidified mixture is cooled and the crystalline precipitate is filter and washed with cold water.
The tan crystals of protocatechuic acid melt at 196–198 °C and the yield of crude protocatechuic acid is 89%.
The ratio of sodium hydroxide to potassium hydroxide is not critical, as long as the total amount of alkali is greater than 7 moles. Mixtures with 10–60% sodium hydroxide become liquid at 120–130 °C. Higher percentages of sodium hydroxide may produce a darker protocatechuic acid, but yields are not significantly affected until the sodium hydroxide concentration reaches 70%.
Sulfur dioxide prevents the formation of a dark-colored product when the reaction mixture is acidified with a strong acid.
The crude crystals have light tan and are suitable for most purposes. It can be further purified by recrystallization from hot water, using the ratio 3:1 of water per acid and 1:10 of activated charcoal per acid. The recrystallized acid is cream-colored and melts at 199–200°C.
3.2. Baterial fermentation
Protocatechuic acid can be produced by various strains of bacteria. For instance, Corynebacterium glutamicum strains have been cultured in a medium supplemented with glucose to produce it. Similarly, Escherichia coli strains such as DH5α, BL21 (DE3), and ATCC 321882 have been propagated in LB medium to produce 3,4-dihydroxybenzoic acid.
To produce protocatechuic acid, Brevibacterium or Corynebacterium strains that cannot use protocatechuic acid as a sole carbon source is mutated. The strains were cultured on complete medium, then replicated on minimum agar medium containing glucose and protocatechuic acid as the sole carbon source.
A colony that could grow on glucose but not protocatechuic acid was isolated to obtain Brevibacterium lactofermentum AJ12106 and Corynebacterium acetoacidophilum AJ12108. Aerobic cultivation of these strains can produce a large amount of protocatechuic acid.
3.3. Extratction from natural sources
In addition to bacterial production, protocatechuic acid can also be isolated from various natural sources. It can be found in the stem bark of Boswellia dalzielii and in the leaves of Diospyros melanoxylon. It is also a major metabolite of antioxidant polyphenols found in green tea. Protocatechuic acid can also be extracted from a variety of plants, including green tea, bamboo leaves, and various fruits and vegetables.
The production of 3,4-dihydroxybenzoic acid is a complex process that involves the breakdown of larger molecules into smaller ones. This process is facilitated by enzymes, which act as catalysts to speed up the reaction. The resulting 3,4-dihydroxybenzoic acid can then be extracted and purified for use in various application.
4. Uses of 3,4-Dihydroxybenzoic Acid
3,4-Dihydroxybenzoic acid (PCA) has a wide range of uses due to its various biological effects1.
- Food and beverage industry: PCA can be used as a natural antioxidant and preservative in food and beverages. For example, it is added to some teas and juices to extend their shelf life.
- Pharmaceutical industry: protocatechuic acid is being investigated as a potential therapeutic agent for a variety of diseases, including cancer, Alzheimer’s disease, and inflammatory bowel disease. Preclinical studies have shown that it has anti-tumor effects, protects against neurotoxicity, and reduces inflammation. It is also being investigated as a potential treatment for COVID-19.
- Cosmetics industry: protocatechuic acid is used in some cosmetics products for its antioxidant and anti-aging properties. For example, it is added to some sunscreens and moisturizers.
- Agricultural industry: protocatechuic acid is being investigated as a potential pesticide and fungicide. Studies have shown that it can inhibit the growth of certain plant pathogens.
Emerging Research
In addition to the potential uses listed above, protocatechuic acid is also being investigated for its potential role in the treatment of other diseases and conditions, including:
Obesity: protocatechuic acid has been shown to reduce body weight and fat mass in animal studies.
Diabetes: protocatechuic acid has been shown to improve blood sugar control and reduce insulin resistance in animal studies.
Cardiovascular disease: protocatechuic acid has been shown to reduce cholesterol levels and improve blood vessel function in animal studies.
Neurodegenerative diseases: PCA has been shown to protect against neurotoxicity and improve cognitive function in animal studies.
Protocatechuic acid is a promising compound with a wide range of potential benefits. Human clinical trials are needed to confirm the safety and efficacy of PCA for the treatment of various diseases and conditions.
5. Toxicology of 3,4-Dihydroxybenzoic Acid
Protocatechuic acid is generally considered safe for consumption. The LD50 of protocatechuic acid in rats is 5000 mg/kg. However, high doses of 3,4-dihydroxybenzoic acid may cause gastrointestinal upset and other side effects.
3,4-Dihydroxybenzoic acid can cause severe eye irritation. It can also irritate the respiratory tract and cause skin irritation. Therefore, precautions should be taken when handling it to avoid direct contact with the eyes, skin, and respiratory tract.
References
- Hydroxycarboxylic Acids, Aromatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a13_519
- PROTOCATECHUIC ACID. – http://www.orgsyn.org/demo.aspx?prep=CV3P0745
- Production of protocatechuic acid by fermentatio. – https://patents.google.com/patent/JPS6098989A/en
- Production of protocatechuic acid by Corynebacterium glutamicum expressing chorismate-pyruvate lyase from Escherichia coli.
- https://en.wikipedia.org/wiki/Protocatechuic_acid
