Phthalimide: Properties, Reactions, Production and Uses
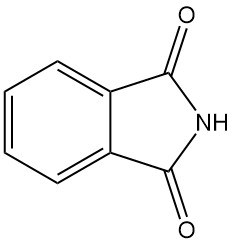
What is Phthalimide?
Phthalimide, also known as 1,3-dioxoisoindoline, is an organic compound with the chemical formula C8H5O2N. It is a white solid that is slightly soluble in water and soluble in basic solutions.
Table of Contents
1. Physical Properties of Phthalimide
Phthalimide is a heterocyclic compound that forms white needles or prisms when crystallized from solution and platelets from sublimation.
It has limited solubility in water (0.3 g at 20 °C, 0.9 g at 50 °C, and 2.2 g at 100 °C per 100 g of water). However, it readily dissolves in acetic acid, sodium hydroxide solution, and potassium hydroxide solution.
Phthalimide sublimes when heated above its melting point. This property makes its purification easier.
Some of the physical properties of phthalimide are listed in the following table:
| Property | Value |
|---|---|
| CAS Number | [85-41-6] |
| Formula | C8H5NO2 |
| Molecular Weight | 147.14 g/mol |
| Melting Point | 238 °C |
| pKa | 8.30 |
| Heat of Combustion | 3560 kJ/mol |
| Heat of Fusion | 187.6 J/g |
| Specific Heat Capacity (100 °C) | 1.21 J g-1 K-1 |
| Vapor Pressure | |
| at 120 °C | 0.10 mbar |
| at 150 °C | 0.95 mbar |
| at 180 °C | 5.93 mbar |
| at 220 °C | 30.7 mbar |
| at 254 °C | 187.6 mbar |
| Flash Point | 214 °C |
| Ignition Temperature | 530 °C |
2. Reactions of Phthalimide
Phthalimide reacts with bases to form water-soluble salts. These salts can then undergo further reactions with halogens (Cl2, Br2, and I2) to yield the corresponding N-chloro, N-bromo, or N-iodo derivatives of phthalimide.
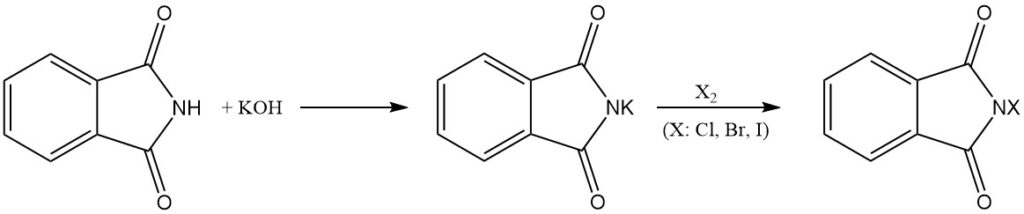
N-halo derivatives can also be obtained by treating alkali metal phthalimides (e.g., potassium phthalimide) with hypochlorous (HOCl) or hypobromous (HOBr) acid.
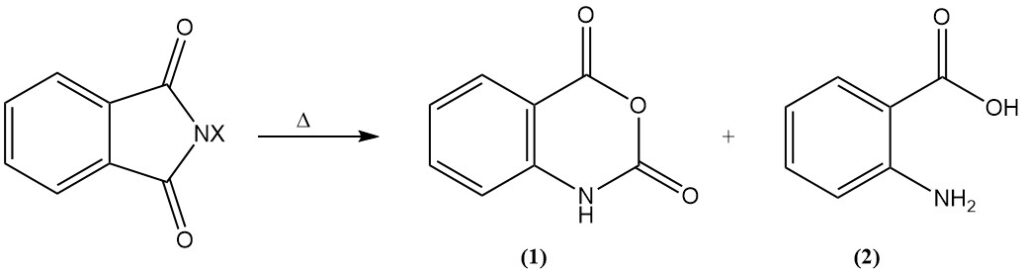
Upon heating, the N-halo derivatives undergo Hofmann degradation, resulting in the formation of either isatoic anhydride (1) or anthranilic acid (o-aminobenzoic acid) (2).
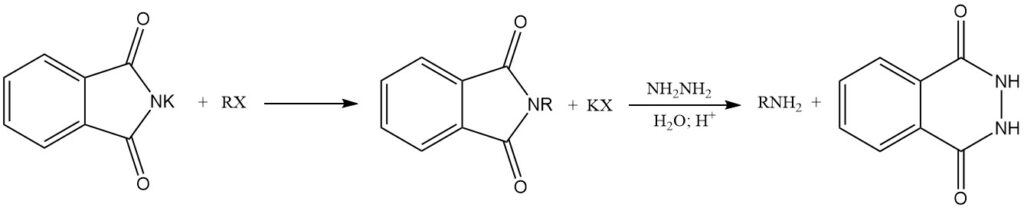
The reaction of alkali metal phthalimides with alkyl halides (RX) yields N-alkylphthalimides. Subsequent hydrolysis or treatment with hydrazine (hydrazinolysis) of these N-alkylphthalimides forms primary amines. This reaction sequence is known as the Gabriel synthesis.
3. Industrial Production of Phthalimide
Phthalimide is primarily synthesized from phthalic anhydride and ammonia, although alternative methods using urea or o-xylene ammoxidation exist.
3.1. Production of Phthalimide from Phthalic Anhydride and Ammonia
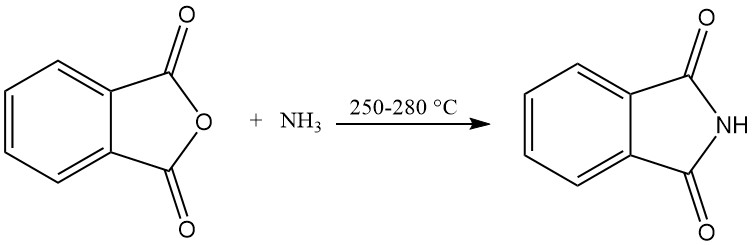
Continuous processes are used in industrial-scale phthalimide production.
The vertical reaction tube process uses an externally heated vertical reaction tube filled with packing material. Molten phthalic anhydride and excess ammonia are continuously fed into the top of the tube and react at 250–280 °C.
The reaction gases are then cooled in a sublimation chamber, where solid phthalimide deposits and is discharged. Excess ammonia and water are removed through an exhaust gas pipe. This process yields phthalimide with a purity of 99% and a high yield of 98%.
In the countercurrent process, molten phthalic anhydride is continuously fed to the top of a reactor, while ammonia is continuously fed to the bottom. The temperature increases gradually from around 150 °C at the top to a maximum of 270 °C at the bottom.
Molten phthalimide with a purity of 99% exits the reactor and is cooled and flaked. It can be dissolved in an aqueous alkali solution for further use.
The exhaust gas from the reactor head, containing water vapor, sublimed phthalimide, and unreacted components, is scrubbed with molten phthalimide from the reactor bottom in a countercurrent fashion. This scrubbing process purifies the exhaust and allows for the recovery of unreacted starting materials.
3.2. Production of Phthalimide from Phthalic Anhydride and Urea
Phthalimide can be produced from phthalic anhydride and urea in a solvent-free process or in a solvent.
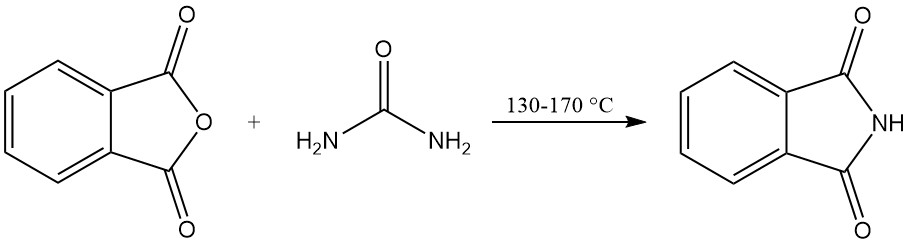
In the solvent-free process, a mixture of phthalic anhydride and urea is heated in a sealed vessel. The reaction proceeds at 130–140 °C, with carbon dioxide and water vapor as byproduct gases. The temperature rises to around 160 °C due to the exothermic reaction.
The reaction is completed when the gas evolution ceases. The solidified reaction mixture is then cooled, ground, and used directly without further purification. This method offers high yields, exceeding 90%.
In the process using a solvent, the solvent is typically a substituted or unsubstituted hydrocarbon, aromatic compound, or heteroaromatic compound (e.g., n-propylbenzene, cumene, 1,2-dichlorobenzene, picoline).
The solvent needs to be chosen such that urea is insoluble while phthalic acid or anhydride has limited solubility. The reaction occurs below the solvent’s boiling point (typically 160–170 °C). After completion, the pure phthalimide product is isolated by filtration and water washing, achieving yields of 95–100%.
3.3. Production of Phthalimide from o-Xylene
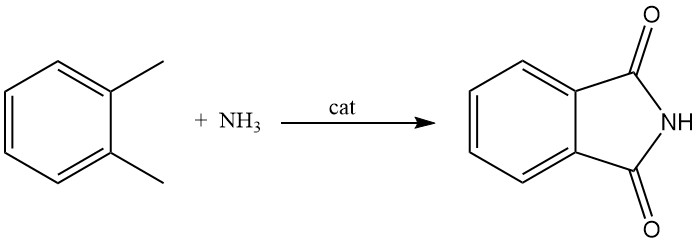
While less common than the methods using phthalic anhydride, phthalimide can also be produced from o-xylene. This process involves reacting o-xylene in the gas phase with ammonia in the presence of a metal oxide catalyst. This catalyst also acts as an oxygen donor.
Depending on the reaction conditions and control, this process can selectively produce phthalimide, phthalamide, or phthalonitrile.
4. Uses of Phthalimide
Phthalimide is used as a raw material in several industries. It is a precursor for the production of anthranilic acid by Hofmann degradation and is used in the Gabriel synthesis to produce a wide range of primary amines.
Phthalimide is used as a protecting group in organic chemistry, particularly peptide synthesis. By reacting with the amino group, it prevents unwanted reactions while the rest of the molecule can be transformed. It can be selectively removed to give the free amine.
It is also utilized as an intermediate in the production of agricultural pesticides, wood preservatives, certain pigments, and pharmaceuticals such as Thalidomide, Amphotalide, Taltrimide, Talmetoprim, and Apremilast.
5. Toxicology of Phthalimide
While data on phthalimide toxicity is limited, available information suggests it has low toxicity.
Phthalimide is broken down in the body into phthalic acid and ammonia.
Studies with rats and mice indicate low acute oral toxicity (LD50 > 5000 mg/kg). No skin or eye irritation was observed in rabbit studies.
Inhalation studies in rats at various concentrations did not reveal any substance-related adverse effects. Long-term studies are lacking.
Ames testing did not show any genotoxic (DNA damaging) effects.
No reliable data on reproductive toxicity is available.
References
- Phthalic Acid and Derivatives, Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a20_181.pub2
- PRACTICAL CONSIDERATIONS IN PREPARATION OF AMINES. – https://www.sciencedirect.com/science/article/abs/pii/B9780080119137500136
- https://go.drugbank.com/categories/DBCAT000722
