Phloroglucinol: production, reactions and uses
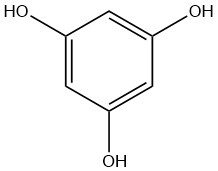
Phloroglucinol, also known as 1,3,5-trihydroxybenzene, is an organic compound with the chemical formula C6H6O3. It is colorless to beige solid at room temperature and is soluble in water, ethanol, and diethyl ether.
HLASIWETZ first identified phloroglucinol in the hydrolysis products of phloretin, which was derived from the bark of fruit trees, in 1855.
The term phloroglucinol is derived from the Greek language and means “sweet bark.” Its derivatives, such as flavones, anthocyanides, and xanthins, are commonly found in plants.
Table of Contents
1. Production of Phloroglucinol
There are several methods for the preparation of phloroglucinol.
One method involves the oxidation of 2,4,6-trinitrotoluene with sodium dichromate to produce 2,4,6-trinitrobenzoic acid which is then decarboxylated.
The resulting product is reduced using iron and hydrochloric acid to form 1,3,5-triaminobenzene.
Phloroglucinol is then obtained by subsequent nucleophilic substitution of the amino groups with hydroxyl groups. However, the disposal of the acidic filtrates containing chromium and iron is a challenge.

During the 1980s, a new method was developed for the synthesis of phloroglucinol from 1,3,5-triisopropylbenzene through the formation of the corresponding trihydroperoxide intermediate.
The addition of hydrogen peroxide to the reaction mixture during the acidolysis step was found to improve the yield of the reaction.

Phloroglucinol can be synthesized by the Beckmann rearrangement of triacetylbenzene trioxime, followed by hydrolysis.
In another method, benzene-1,3,5-tricarboxylic acid triamide undergoes Hofmann rearrangement.
Additionally, phloroglucinol can be obtained by the reaction of 1,3,5-tribromobenzene or hexachlorobenzene with an alkoxide, followed by acidolysis. Another alternative involves the hydrolysis of 4-chlororesorcinol with potassium hydroxide.
2. Chemical Reactions of Phloroglucinol
Due to its keto-enol tautomerism, phloroglucinol is capable of reacting to form both triphenol and triketone derivatives. The molecule displays triketone behavior in reactions such as with hydroxylamine to form the corresponding trioxime, and with sodium bisulfite to produce mono-, di-, and tri-substituted products.
In contrast, triphenol behavior is demonstrated in reactions such as etherification and esterification, which lead to the formation of mono-, di-, and tri-substituted phenols.
Phloroglucinol can also undergo Friedel-Crafts alkylation and acylation, and nitration.
Additionally, phloroglucinol is known to act as a reducing agent. When present in an alkaline aqueous solution, it can absorb gaseous oxygen, though this occurs at a slower rate than with pyrogallol.
Phloroglucinol can reduce Fehling’s solution as well as various precious metal ions, including Au2+, Ag+, and Pt2+. When subjected to basic conditions and treated with methyl iodide, phloroglucinol undergoes ring methylation.
Other reactions of phloroglucinol include its reaction with aqueous ammonia to form either 5-aminoresorcinol or 3,5-diaminophenol. Upon heating, phloroglucinol forms phloroglucide.
Hydrogenation of phloroglucinol produces 1,3,5-trihydroxycyclohexane. Coupling with diazonium salts is also a readily achievable reaction.
3. Uses of Phloroglucinol
Phloroglucinol serves as a coupler in diazotyping where it reacts with a diazo compound to form a high molecular weight molecule which has a black color.
Both the diazo and hydroxy compounds are present in the coating during the drying process.
Additionally, phloroglucinol is used in photocopying processes.
In medicine, it is used in the treatment of painful spasms of digestive origin (spastic colitis), biliary (hepatic colic), urological (renal colic) and gynecological (painful periods and contractions of the uterus during pregnancy).
References
- Phenol Derivatives; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a19_313
- https://www.vidal.fr/medicaments/gammes/phloroglucinol-arrow-31619.html
