Acetylation
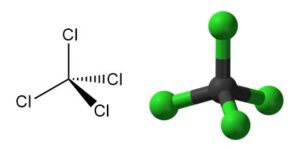
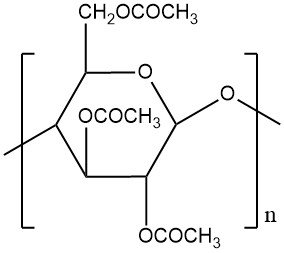
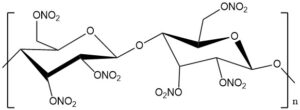
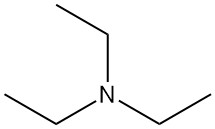
In chemistry, acetylation is an organic reaction in which an acetyl group (CH3CO-) is introduced into a molecule. This reaction typically involves the use of acetic acid (CH3COOH) or acetic anhydride (CH3CO)2O as the acetylating agent. The resulting product is called acetate.
Read MoreAcetylation