Ethylamine: Properties, Reactions, Production and Uses
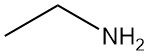
What is Ethylamine?
Ethylamine, also known as ethanamine, is a primary aliphatic amine with the chemical formula C2H5NH2. It is a flammable gas at room temperature with an ammonia-like odor.
Ethylamine can be found naturally in fruits and vegetables, grains, coffee, cheeses, and seafood. It is also a component of tobacco smoke.
Table of Contents
1. Physical Properties of Ethylamine
Ethylamine is a colorless, flammable liquid or gas, depending on the ambient temperature. It is a weak base that has an ammonia-like odor with a reported odor awareness threshold in a range between 0.027 ppm and 3.5 ppm. It is miscible with water, ethanol, and ether.
The combustion of ethylamine produces toxic nitrogen oxides. Commercially, ethylamine is sold as a 70% aqueous solution or as a compressed gas.
The physical properties of ethylamine are listed in the following table:
| Property | Value |
|---|---|
| CAS number | [75-04-7] |
| Chemical formula | C2H5NH2 |
| Molecular mass | 45.09 g/mol |
| Melting point | -80.6 °C |
| Boiling point | 16.6 °C |
| Density at 20 °C | 0.6829 g/cm3 |
| pKb at 25 °C | 3.25 |
| Refractive index | 1.3663 |
| Vapor Density | 1.56 |
| Vapor pressure, at 20 °C | 121 kPa |
| Flash point | -52 °C |
| Autoignition Temperature | 385 °C |
| Heat of Combustion at 25 °C (liquid) | -1713.3 kJ/mol |
| Heat of Vaporization | 6,845.1 g cal/g.mol |
| Surface Tension at 25 °C | 19.20 mN/m |
2. Reactions of Ethylamine
Ethylamine is a weak primary amine that dissolves in water to form a basic solution. It is a stronger base than ammonia that reacts with organic or inorganic acids to form salts that are very soluble in water but insoluble in organic solvents.
For example, the reaction of ethylamine with hydrochloric acid produces ethyl ammonium chloride, and with propanoic acid forms ethyl ammonium propionate.
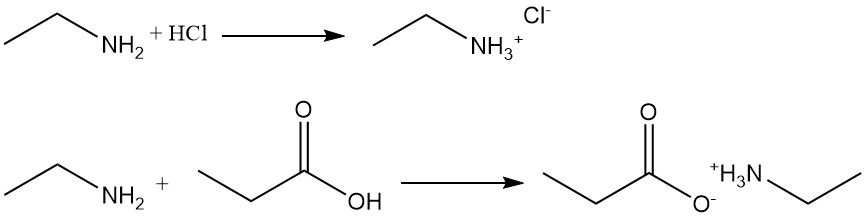
Ethylamine reacts with carboxylic acids and their esters, chlorides, and anhydrides to give the corresponding amides. The reaction with carboxylic acid chlorides is a highly exothermic reaction that gives very good yields. The reaction with carboxylic acids often forms ammonium salt, which needs heating to produce the amides.
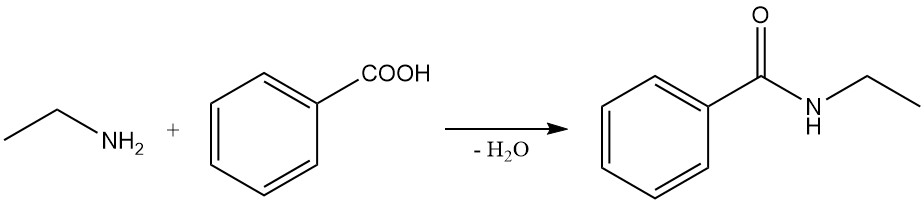
Examples of these reactions include the reaction of benzoic acid and ethylamine to form N-ethylbenzamide and the reaction of ethylamine with succinic anhydride to produce 1-ethylpyrrolidine-2,5-dione and N1,N4-diethylsuccinamide.
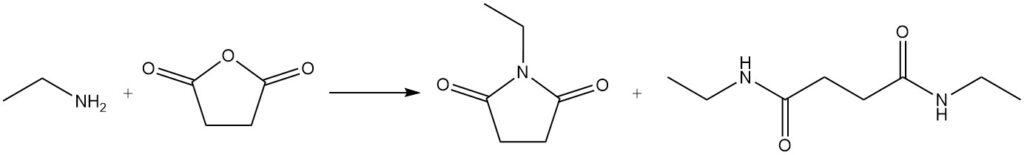
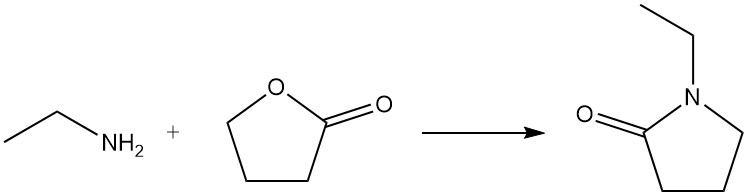
When ethylamine reacts with a lactone, a lactam is formed; for example, 1-ethyl-2-pyrrolidone is prepared from ethylamine and γ-butyrolactone.
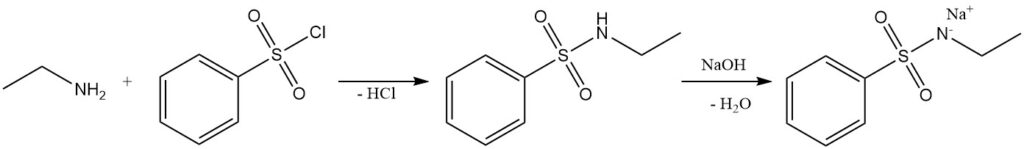
The reaction of ethylamine with benzenesulfonyl chloride (Hinsberg test) forms the alkali-soluble N-ethyl benzenesulfonamide.
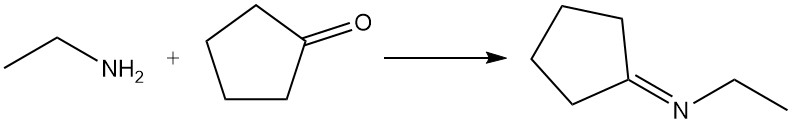
Depending on the reaction conditions, aldehydes and ketones react with ethylamine to form imines (Schiff bases) or enamines; these products can be hydrogenated to give more highly alkylated amines. Aldehydes generally react faster than ketones. A common example is the reaction of ethylamine and cyclopentanone to produce N-ethylcyclopentanimine.
Ethylcyclohexylamine, an important amine used in many industries, is produced by the reaction of ethylamine with cyclohexanone followed by hydrogenation.
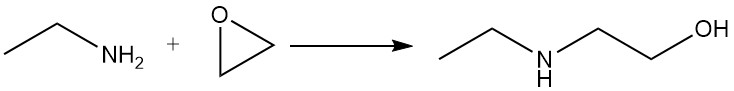
Ethylamine reacts with epoxides to give a mixture of mono- and dioxyethyl derivatives.
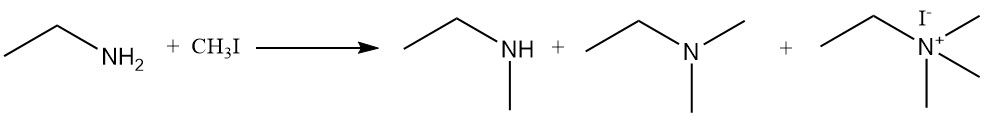
The reaction of ethylamine with alkyl halides and dialkyl sulfates produces a mixture of secondary and tertiary amines and quaternary ammonium compounds. One example is the reaction of ethylamine and methyl iodide to form a mixture of N-methylethanamine, N,N-dimethylethanamine and N,N,N-trimethylethyl ammonium iodide.
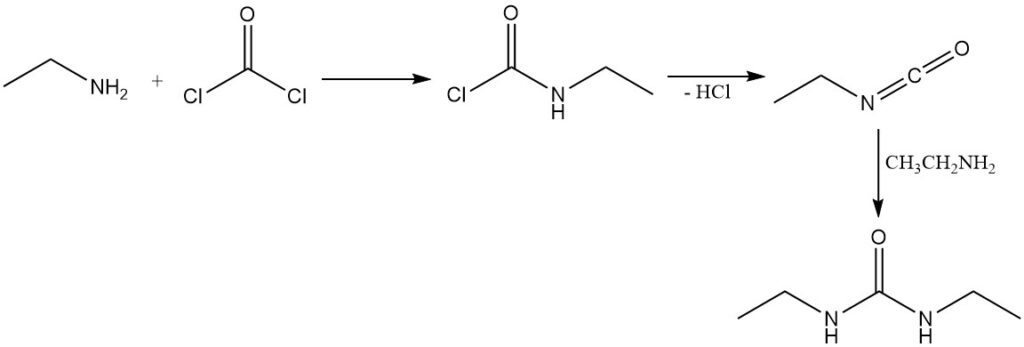
The reaction of phosgene with ethylamine leads first to the formation of ethylcarbamic chloride; subsequent elimination of the hydrogen chloride gives isocyanatoethane. Excess ethylamine then reacts with isocyanatoethane to produce 1,3-diethylurea.
Ethylamine reacts with chloroform under basic conditions (potassium or sodium hydroxide) to form ethylisonitrile.

The addition of ethylamine to acrylonitrile forms 3-(ethylamino)propanenitrile, which can be readily hydrogenated to the coresponding N-ethyl-1,3-propanediamine.

The oxidation of ethylamine with hydrogen peroxide using tungsten-doped zeolites as catalysts produces acetaldehyde oxime.
The dehydrogenation of ethylamine produces acetonitrile.
3. Production of Ethylamine
3.1. Production of Ethylamine from Ammonia and Ethanol
The catalytic reaction of ethanol with ammonia is the predominant method for ethylamine synthesis. This process concurrently generates diethylamine and triethylamine as byproducts due to the reactivity of ethylamine with ethanol.
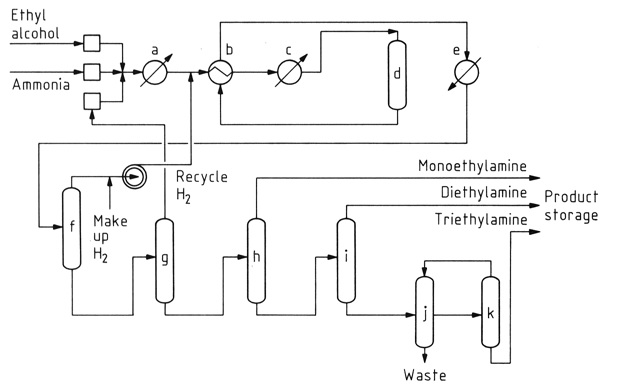
a) Vaporizer; b) Heat exchanger; c) Superheater; d) Catalytic converter; e) Product cooler; f) Gas separator; g) Ammonia column; h) Monoethylamine column; i) Diethylamine column; j) Decanter; k) Triethylamine column
While the initial conversion of ethanol to ethylamine is nearly energy-neutral, the formation of secondary and tertiary amines is exothermic, which favors their thermodynamic production. Reaction conditions, including temperature, ammonia excess, and residence time, influence product distribution.
Historically, dehydration catalysts (e.g., alumina, silica) were used, but recently, metal-based catalysts have become predominant. Nickel, cobalt, copper, and iron catalysts are commonly employed, with platinum and palladium being less selective due to potential C-C or C-N bond cleavage during the reaction.
Promoters such as silver, zinc, indium, manganese, molybdenum, and alkali metals enhance catalyst performance. These active components are typically supported on alumina, silica, or zirconia, with zeolites showing higher selectivity for primary amine.
This reaction occurs in a fixed-bed reactor at 0.5–25 MPa and 100–250 °C, depending on catalyst type and phase (liquid or gas).
The mechanism involves ethanol dehydrogenation to acetaldehyde as the rate-determining step. Subsequent ammonia addition, water elimination, and imine or enamine formation are followed by hydrogenation to ethylamine.
Side reactions include amine disproportionation (especially at higher temperatures), aldol condensation of intermediate aldehydes, Schiff base formation, and nitrile formation under specific conditions.
A two- to eightfold ammonia excess shifts equilibrium towards ethylamine formation.
3.2. Production of Ethylamine from Acetaldehyde
Acetaldehyde, readily accessible from oxo synthesis, is a preferred precursor to ethylamine compared to ethanol due to economic advantages. This process involves a two-step reaction: initial formation of an imine (Schiff base) from acetaldehyde and ammonia, followed by hydrogenation to ethylamine.

This process employs a fixed-bed catalyst and a reaction mixture containing acetaldehyde, ammonia, and hydrogen. Alternatively, a two-stage process can be beneficial, separating imine formation and subsequent hydrogenation.
Vapor-phase operation at 100–160 °C and atmospheric or slightly elevated pressure is common. High-pressure conditions may be used with effective heat removal systems, such as high recycle rates or multitubular reactors. Lower reaction temperatures compared to alcohol amination could potentially achieve higher selectivity.
Catalysts are generally similar to those used in alcohol amination. The crude ethylamine is purified by distillation.
While alternative reducing reagents exist, hydrogen remains the primary industrial choice due to handling and cost considerations.
3.3. Other Processes
Ethylamine can be produced by the addition of ammonia to ethylene using acidic zeolite catalysts such as H-mordenite. Although the conversion for the direct amination of ethylene is low, being limited by kinetic factors and the thermodynamic equilibrium, the selectivity for monoethylamine is excellent.
Ethylamine can also be prepared selectively by electroreduction of acetonitrile at ambient temperature and pressure using Cu nanoparticles as an electrocatalyst. However, this method is not industrially important.
4. Uses of Ethylamine
Ethylamine is primarily used as a precursor for triazine herbicides by reaction with cyanuric chloride. Atrazine, the predominant triazine herbicide, is employed for broadleaf and grassy weed control, primarily in corn and grain sorghum cultivation. Simazine, another ethylamine-derived triazine, is used in citrus and sugarcane agriculture.
Beyond herbicides, ethylamine is used in the synthesis of various chemicals. It reacts with methallyl chloride to form ethylmethallylamine, a key intermediate in dinitroaniline herbicide ethalfluralin production. Additionally, ethylamine serves as a precursor to ethylcyclohexylamine, which is subsequently converted to the thiocarbamate herbicide cycloate.
It is also used in the resin industry, petroleum refining, and the production of pharmaceuticals, corrosion inhibitors, polyurethane catalysts, and textile agents.
Moreover, ethylamine is employed as a solvent extraction agent, dye intermediate, rubber latex stabilizer, and laboratory reagent. It is an intermediate in the preparation of detergent, photographic dye, emulsifiers, and paint removers. Ethylamine has been identified as a human metabolite.
The demand for atrazine is declining due to environmental concerns, which have led to bans in certain regions.
5. Toxicology of Ethylamine
Ethylamine is a volatile, alkaline compound readily absorbed through dermal and respiratory routes. Despite its efficient urinary excretion following partial metabolism, exposure can induce adverse health effects. Its primary toxic action is attributed to its corrosive properties.
Acute and Short-Term Toxicity
Ethylamine is a severe irritant to the skin, eyes, and respiratory tract. Prolonged contact results in corrosive tissue damage.
Acute oral LD50 in rats is 400 mg/kg, while dermal LD50 in rabbits is 270 mg/kg. Inhalation LC50 in rats is 5540 ppm (1-hour exposure). Dermal application of a 70% aqueous solution to guinea pigs caused severe skin burns and necrosis.
Ocular instillation of a 1% solution in rabbits induced severe irritation. Short-term inhalation of 50 ppm caused corneal irritation in rabbits.
Industrial exposures have resulted in eye irritation and corneal edema in humans. Reports of transient visual disturbances (glaucopsia) similar to those caused by triethylamine exposure exist, though the causal link to ethylamine is unclear.
Chronic Toxicity
Rabbits exposed to 50 ppm ethylamine show lung damage and corneal injury. A higher concentration of 100 ppm induced kidney damage. Rats have less sensitivity, with only minor irritation at 100 ppm. Nasal irritation and damage occurred at 500 ppm following 24 weeks of exposure.
Long-term studies evaluating carcinogenic potential are absent. Fetal development studies are also unavailable.
No data exists on chronic ethylamine exposure in humans.
Genotoxicity
Positive results were observed in in vitro cell culture genotoxicity assays. Ethylamine did not alter DNA synthesis in rodents.
Ecotoxicity
Ethylamine exhibits moderate to low acute toxicity to aquatic organisms, with LC50 values ranging from 40 to 1000 ppm for goldfish and EC50 values of 100 to 200 ppm for Daphnia magna.
Other Hazards
Ethylamine is highly flammable and can form explosive vapor mixtures. It is a severe irritant to the eyes, nose, and respiratory tract.
References
- Amines, Aliphatic. Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a02_001.pub2
- Ethylamine. – https://www.sciencedirect.com/science/article/abs/pii/B9780123864543005042
- https://www.sciencedirect.com/science/article/abs/pii/B9780128243152001949
- https://patents.google.com/patent/US20070112218A1/en
- https://www.nature.com/articles/s41467-021-22291-0
- https://products.basf.com/global/en/ci/ethylamine-solution-70
