Cyclohexylamine: Properties, Reactions, Production and Uses
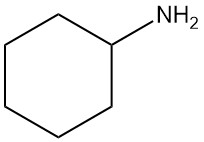
What is Cyclohexylamine?
Cyclohexylamine, also known as aminocyclohexane, is an aliphatic primary amine with the chemical formula C6H13N. It is a colorless liquid that is miscible with water and common organic solvents.
Table of Contents
1. Physical Properties of Cyclohexylamine
Cyclohexylamine is a colorless liquid with an ammonia or fishy odor that is very soluble in ethanol, ether, acetone, and common organic solvents. It forms an azeotrope with water that boils at 96.4 °C and contains 44.2% cyclohexylamine.
The physical properties of cyclohexylamine are listed in the following table:
| Property | Value |
|---|---|
| CAS number | [108-91-8] |
| Chemical formula | C6H13N |
| Molecular weight | 99.18 g/mol |
| Melting point | -17.8 °C |
| Boiling point | 134.5 °C |
| Density | 0.8647 g/cm3 |
| Refractive index | 1.4592 |
| Viscosity at 20 °C | 2.10 Pa·s |
| pKa | 10.63 |
| Vapor Density | 3.42 |
| Vapor pressure | 1.4 kPa at 20 °C |
| Specific heat capacity at 20 °C | 2.366 J g-1K-1 |
| Heat of vaporization | 399.86 J/g |
| Flash point (closed cups) | 26.5 °C |
| Ignition temperature | 265 °C |
| Ignition range in air | 1.6–9.4 vol% |
2. Chemical Reactions of Cyclohexylamine
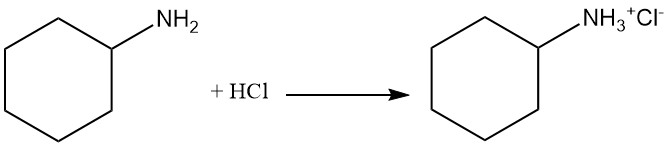
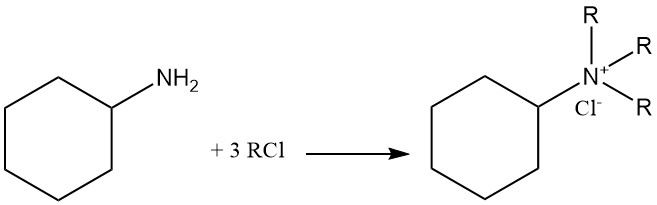
Cyclohexylamine is a weak base with a pKb of 3.4 that has similar reactivity of primary aliphatic amines. It forms salts with both Brønsted and Lewis acids. Exhaustive alkylation leads to quaternary ammonium cation formation.
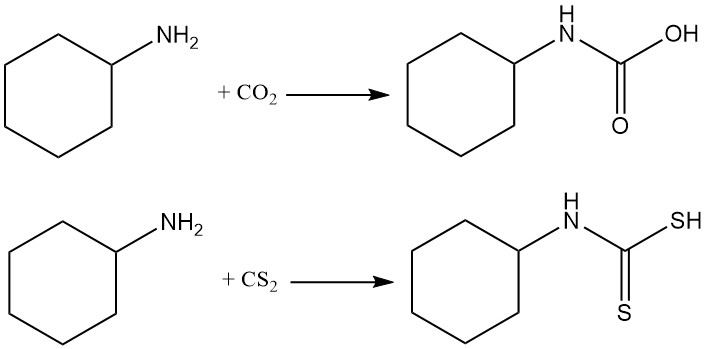
Cyclohexylamine reacts with carbon dioxide and carbon disulfide to produce carbamates and thiocarbamates, respectively.
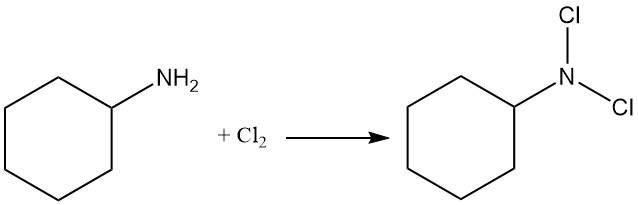
The reaction of cyclohexylamine with chlorine produces N,N-dichlorocyclohexylamine.
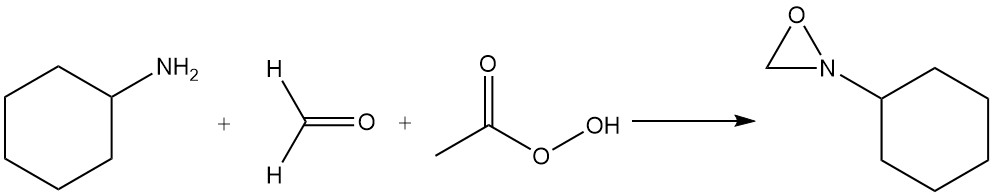
2-Cyclohexyloxaziridine is prepared by reacting of a mixture of cyclohexylamine, formaldehyde, and peracetic acid.
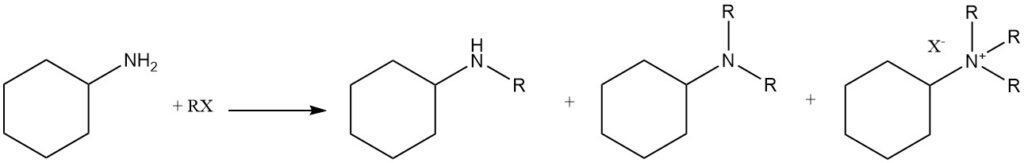
N-alkylation of cyclohexylamine can be achieved by using various alkylating agents, including alkyl halides, sulfates, and phosphates. Alternatively, alcohols can be used as alkylating compounds in the presence of catalytic metals (aluminum, copper, nickel, cobalt, or platinum) or under Leuckart-Wallach conditions.
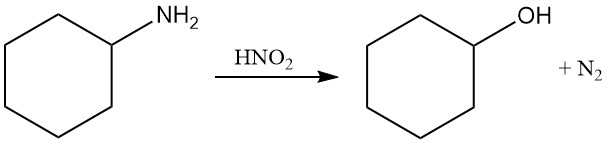
Cyclohexylamine undergoes diazotization with nitrous acid to form nitrogen gas and cyclohexanol.
Acid chlorides react with cyclohexylamine under Schotten-Baumann conditions to form cyclohexyl amides.
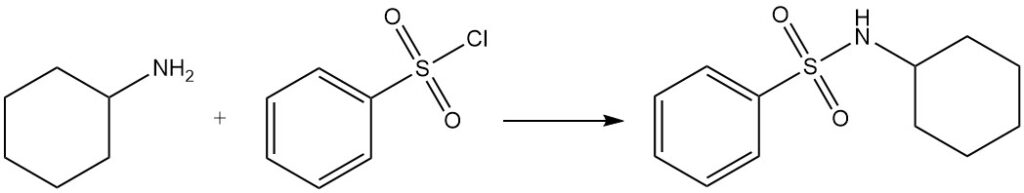
Benzenesulfonyl chloride reacts with cyclohexylamine to produce N-cyclohexylbenzenesulfonamide, which is soluble in alkaline solutions (Hinsberg test).
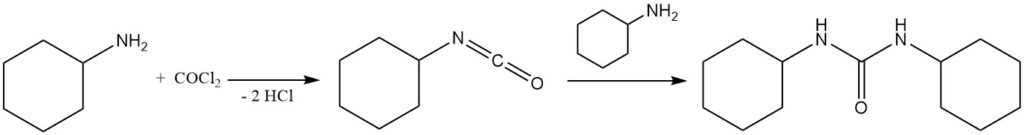
Phosgene reacts with cyclohexylamine to form isocyanatocyclohexane, which further reacts with cyclohexylamine to form 1,3-dicyclohexylurea.
Because of the nucleophilic character of cyclohexylamine, it reacts with epoxides to produce hydroxyalkyl and dihydroxyalkyl amine derivatives.
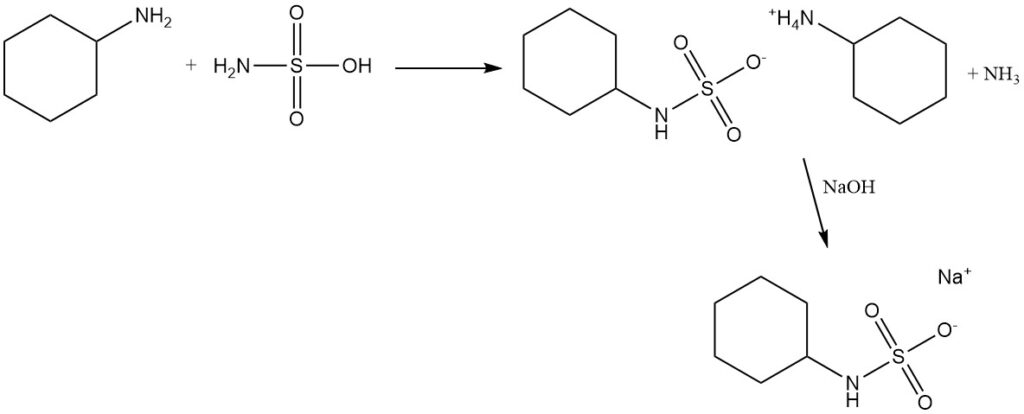
High-purity cyclohexylamine reacts with sulfamic acid, followed by treatment with sodium hydroxide or calcium hydroxide, to form sodium cyclohexylsulfamate or calcium cyclohexylsulfamate, which are artificial sweeteners used in the past.
The condensation reaction of cyclohexylamine with mercaptobenzothiazole produces N-cyclohexyl-2-benzothiazolesulfenamide, which is used as a moderated rubber accelerator.
3. Industrial Production of Cyclohexylamine
Cyclohexylamine is commercially produced by the reaction of cyclohexanol with ammonia and hydrogen in the vapor phase using metal catalysts such as Ni or Co.
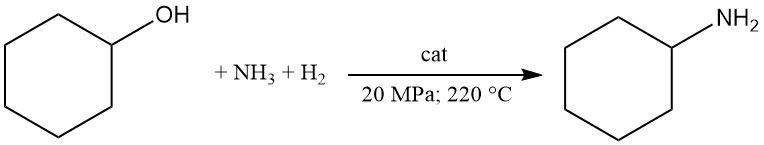
In this process, cyclohexanol reacts at 20 MPa and 220 °C with at least 3 mol of ammonia in the presence of circulating hydrogen over a fixed-bed catalyst.
An increased ammonia-to-cyclohexanol feed ratio favors cyclohexylamine formation and reduces the formation of the byproduct dicyclohexylamine.
Reductive amination of cyclohexanone using a continuous process is another method to produce cyclohexylamine. In this process, pressurized hydrogen and ammonia react with cyclohexanone over nickel or cobalt catalysts at 0.1–20 MPa and high temperatures up to 275 °C.

An ammonia-cyclohexanone ratio of 3.3:1 and a hydrogen-cyclohexanone ratio of 6.5:1 have been claimed.
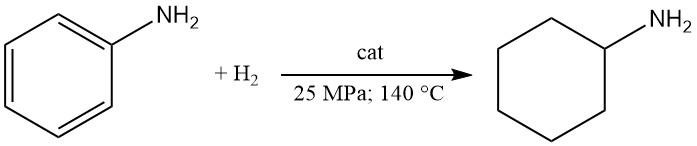
Cyclohexylamine is produced by liquid-phase hydrogenation of aniline over a cobalt-alumina catalyst at 25 MPa and 140 °C with an 80% yield. However, vapor-phase hydrogenation over nickel-on-pumice favors the formation of dicyclohexylamine.
The choice of catalysts and reaction conditions influence the selectivity of the reaction for cyclohexylamine. For example, if aniline is hydrogenated at 160–200 °C in the presence of a ruthenium–palladium catalyst supported on γ-alumina, the final product mixture contains only 19.3% cyclohexylamine and 80.3% dicyclohexylamine.
High selectivity for cyclohexylamine is achieved by adding ammonia during the hydrogenation of aniline at 160 to 180 °C and 2 to 5 MPa using a ruthenium-on-carbon catalyst.
Cyclohexylamine is also manufactured on a commercial scale by hydrogenation of nitrobenzene without intermediate separation of aniline.
Direct cyclohexylamine synthesis from phenol using rhodium or nickel catalysts has been explored, but with lower selectivity towards cyclohexylamine compared to dicyclohexylamine.
Cyclohexylamine is purified from byproducts by distillation under reduced pressure.
4. Uses of Cyclohexylamine
Cyclohexylamine is a water-miscible compound that formsan azeotrope with water. It is used as a corrosion inhibitor in low-pressure steam systems because it forms a protective film and is an acid-neutralizing agent.
In 2000, approximately 55% of US cyclohexylamine production was dedicated to this application. Compared to morpholine, cyclohexylamine offers superior chemical stability in high-pressure environments and don’t form nitrosamine.
It is used as a corrosion inhibitor for radiator alcohol solutions and also in the paper- and metal-coating industries for moisture and oxidation protection.
Beyond corrosion inhibition, cyclohexylamine is used as a vulcanization accelerator, anticorrosive additive, and precursor to various compounds, including plasticizers, emulsifiers, coagulants, and epoxy hardeners.
Its salts with fatty acids prevent foaming in mineral oils. Cyclohexylamine is also used as a chain terminator in polyamide polymerization and as a precursor for herbicides like hexazinone. It also functions as a hardener for epoxy resins and as a catalyst for polyurethanes.
Historically, cyclohexylamine was used in the production of cyclamate sweeteners.
Major producers of cyclohexylamine include US Amines, Air Products, BASF, Borsodchem, Jintian Enterprises, and New Japan Chemical Co.
5. Toxicology of Cyclohexylamine
Cyclohexylamine is an alkaline liquid readily absorbed via oral and respiratory routes, though dermal absorption is slower. Rapid urinary excretion occurs with minimal metabolic alteration. The compound possesses a strong, fish-like odor with an odor threshold around 2.5 mL/m³.
Human exposure to cyclohexylamine can induce sympathomimetic effects at daily doses of 5 mg/kg. Extensive toxicological studies have been conducted due to its association with the sweetener saccharin.
Long-term feeding studies in rodents revealed no carcinogenic, mutagenic, or teratogenic effects at doses up to approximately 20 mg/kg per day. Fertility studies in rats showed no adverse effects, despite minor testicular atrophy at higher doses.
Occupational exposure limits have been established to protect human health. A 4-hour inhalation study in humans showed irritation at 10 mL/m³, while a time-weighted average concentration of 2 mL/m³ was well tolerated.
Consequently, the maximum concentration at the workplace (MAK value) was set at 2 mL/m³ with a peak limitation category of I.
Acute toxicity studies in animals indicate an oral LD50 of 710 mg/kg in rats and a dermal LD50 of 320 mg/kg in rabbits.
References
- Amines, Aliphatic, Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_001.pub2
- Amines, Cycloaliphatic. – https://onlinelibrary.wiley.com/doi/10.1002/0471238961.0325031203011905.a01.pub2
- Aniline and Its Derivatives. – https://onlinelibrary.wiley.com/doi/10.1002/0471238961.0114091201130914.a01.pub2
- Cyclohexylamine [MAK Value Documentation, 2017]. – https://onlinelibrary.wiley.com/doi/10.1002/3527600418.mb10891e6218
