Cyclohexanone: Properties, Reactions, Production and Uses
What is cyclohexanone?
Cyclohexanone, also known as ketohexamethylene or pimelic ketone, is an organic compound with the chemical formula C6H10O. It is a colorless to pale yellow liquid with an odor similar to that of acetone that is very soluble in most organic solvents.
It was first synthesized by the dry distillation of calcium pimelate, and later by Bouveault by the catalytic dehydrogenation of cyclohexanol.
Cyclohexanone is produced industrially on a large scale as a raw material for nylon manufacturing.
Table of Contents
1. Physical Properties of Cyclohexanone
Cyclohexanone is a colorless liquid with an odor similar to that of peppermint and acetone. It is very soluble in methanol, ethanol, acetone, benzene, n-hexane, nitrobenzene, diethyl ether, naphtha, xylene, ethylene glycol, isoamyl acetate, diethylamine, and most organic solvents.
Cyclohexanone can dissolve a variety of products, such as cellulose nitrate, cellulose acetate, cellulose ethers, vinyl resins, raw rubber, waxes, fats, shellac, basic dyes, oils, latex, bitumen, kaure, and elemi. It forms an azeotrope with water that boils at 96.6 °C with a composition of water:cyclohexanone of 56:44.
The physical properties of cyclohexanone are presented in Table 1.
| Property | Value |
|---|---|
| CAS number | [108-94-1] |
| Chemical formula | C6H10O |
| Molecular weight | 98.15 g/mol |
| Melting point | -47 °C |
| Boiling point | 156.4 °C |
| Vapor pressure | 0.52 kPa at 20 °C |
| Density | 0.9455 g/cm3 |
| Refractive index | 1.4552 |
| Expansion coefficient | 9.14 × 10-4 |
| Dynamic viscosity | 1.803 mPa · s at 30 °C |
| Specific heat | 1.811 j/g |
| Surface tension | 33.51 mN/m at 30 °C |
| Heat of fusion | 1.501 kJ/mol |
| Heat of combustion | -3.521 MJ/mol |
| Heat of vaporization | 44.92 kJ/mol |
| Heat of formation | -272 kJ/mol |
| Flash point | 54 °C |
| Autoignition temperature | 420 °C |
| Solubility in water at 20 °C | 9.0 g/100 g of water |
2. Reactions of Cyclohexanone
Due to the presence of a carbonyl group, cyclohexanone undergoes a variety of reactions typical of aliphatic ketones. Approximately 0.02% of the molecule exists in the enol tautomer at ambient temperature.
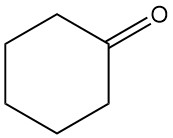
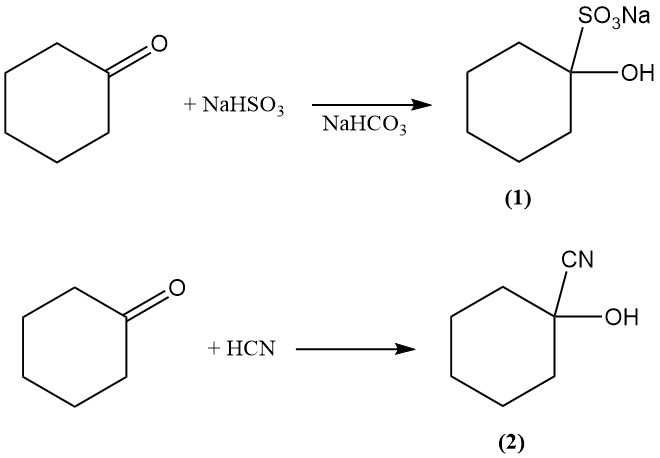
The addition reaction of cyclohexanone with bisulfite and hydrogen cyanide produces 1-hydroxycyclohexane-1-sulfonate (1) and 1-hydroxycyclohexane-1-carbonitrile (2), respectively.
The Grignard reaction forms tertiary alcohols after hydrolysis.
Catalytic hydrogenation or reduction of cyclohexanone using sodium borohydride or lithium aluminum hydride yields cyclohexanol, while more rigorous conditions form cyclohexane.
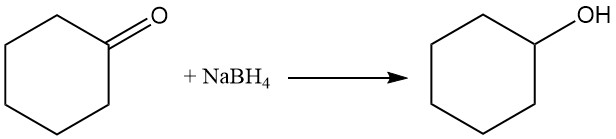
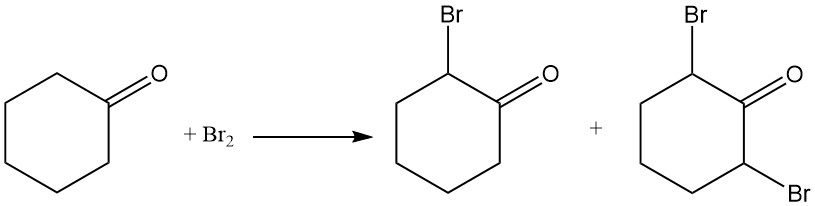
Cyclohexanone reacts with chlorine or bromine to form halogenated compounds at the α-position or at both the 2- and 6-positions.
It can easily undergo base-catalyzed Aldol condensation with itself or with other aldehydes or ketones.
Cyclohexanone forms enamines when reacted with secondary amines.
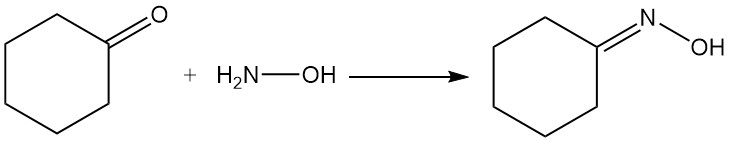
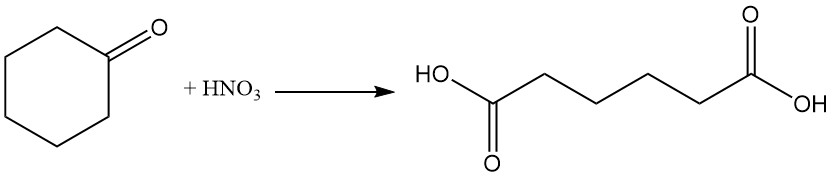
Industrially important reactions of cyclohexanone are cyclohexanone oxime formation by reaction with hydroxylamine and oxidation with nitric acid to adipic acid.
3. Industrial Production of Cyclohexanone
Cyclohexanone is produced commercially by the following major processes:
- Dehydrogenation of cyclohexanol
- Oxidation of cyclohexane
- Hydrogenation of phenol
Most large-scale facilities use cyclohexane oxidation technology to produce a mixture of cyclohexanol and cyclohexanone. DSM in Holland and Allied Signal Corp. in the United States are the only remaining large plants that still use the hydrogenation of phenol.
Catalytic and non-catalytic processes are used to oxidize cyclohexanol to cyclohexanone.
3.1. Production of Cyclohexanone by Hydrogenation of Phenol
Phenol hydrogenation product distribution, including cyclohexanol, cyclohexanone, or their mixture, is dependent on catalyst choice and reaction conditions.
Figure 1 illustrates vapor-phase cyclohexanone production from phenol.
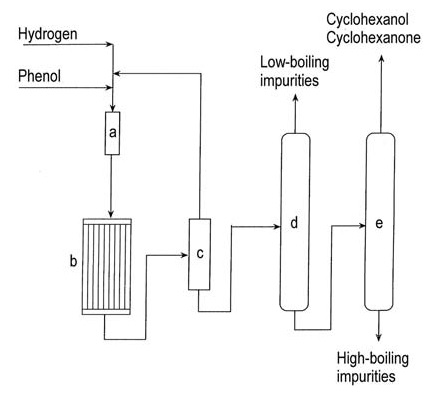
a) Phenol evaporator; b) Hydrogenation reactor; c) Condenser; d) Low-boiler removal column; e) Cyclohexanol/cyclohexanone recovery column
The hydrogenation of phenol to cyclohexanone in the vapor phase uses diverse supported noble metal catalysts, including palladium, platinum, iridium, ruthenium, rubidium, and osmium. The reaction occurs at 140–170 °C under atmospheric pressure and achieves a 95% yield at complete conversion.
Commercial liquid-phase hydrogenation of phenol using palladium on a carbon catalyst yields >99% cyclohexanone at 90% conversion under mild conditions.
3.2. Production of Cyclohexanone by Dehydrogenation of Cyclohexanol
The dehydrogenation of cyclohexanone produces cyclohexanone, which is a caprolactam precursor.
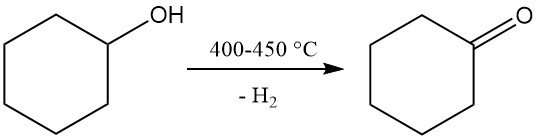
The non-catalytic vapor-phase dehydrogenation of cyclohexanol is carried out at a temperature of 400–450 °C. The generated vapors are cooled, hydrogen is separated, and the crude cyclohexanone, containing cyclohexene and water as byproducts, is purified through distillation. The resulting cyclohexanone purity reaches 98–99%.
Catalytic dehydrogenation of cyclohexanol to cyclohexanone using catalysts like chromium oxide-copper, copper chromate, nickel, zinc sulfide, zinc-iron, cobalt carbonate, and other metals occurs under milder conditions and gives better yields.
3.3. Production of Cyclohexanone by Oxidation of Cyclohexane
The liquid-phase air oxidation of cyclohexane to cyclohexanol and cyclohexanone is usually carried out, either uncatalyzed or with a soluble cobalt catalyst, in a series of agitated reactors or a single tower oxidizer at a temperature of 140–180 °C and a pressure of 0.8–2 MPa.
Cyclohexyl hydroperoxide is an intermediate in this reaction and is converted by the oxidizers to cyclohexanol, cyclohexanone, and byproducts.
When chromium (III) is added to the air oxidizer, it catalyzes the dehydration of cyclohexyl hydroperoxide to cyclohexanone and water, leading to a higher selectivity for cyclohexanone.
Another method to increase the ratio of cyclohexanone to cyyclohexanol in the final product is by decomposing the cyclohexyl hydroperoxide with an aqueous caustic phase containing a few ppm of cobalt.
A flow diagram of this caustic process is shown in Figure 2.
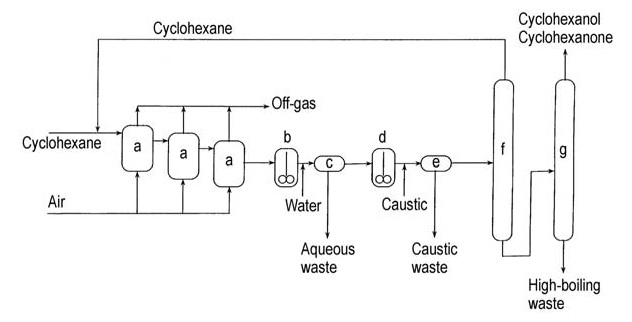
a) Air oxidizers; b) Water wash decanter; c) Decantation; d) Caustic decomposition reactor; e) Caustic decantating; f) Cyclohexane recovery column; g) Cyclohexanol/cyclohexanone recovery column
The crude product in this process contains a mixture of cyclohexanone and cyclohexanol, which can be separated by distillation and converted to cyclohexanone by dehydrogenation to increase the overall yield of cyclohexanone.
4. Uses of Cyclohexanone
Over 97% of all cyclohexanone produced is used as a raw material for the production of adipic acid and caprolactam, which are used in industrial nylon manufacturing.
Cyclohexanone is also used as a solvent for lacquers, resins, polymers, and other compounds. Other uses include as starting material in the synthesis of insecticides, herbicides, and pharmaceuticals.
Cyclohexanone is used in the manufacture of magnetic and video tapes.
Compliance with California Rule 66 allows its use as an isophorone substitute for resin and polymer solvent applications.
5. Toxicology of Cyclohexanone
Cyclohexanone exhibits low toxicity via oral, dermal, and inhalation exposure. Liquid or vapor contact may induce a transient corneal injury. Prolonged or substantial skin contact can cause irritation and defatting.
Inhalation exposure caused lung congestion, edema, and hemorrhage. High vapor concentrations or repeated exposure can lead to CNS depression.
LD50 values ranged from 0.93 to 1.54 g/kg (ip) and 1.8 to 2.1 g/kg (intragastric) in animal studies, with guinea pigs showing highest sensitivity
While both positive and negative genetic activity results exist, carcinogenicity evidence remains inconclusive. Cyclohexanone demonstrated non-teratogenicity in rats and mice.
Two-generation inhalation studies in rats revealed no adverse effects on growth, development, or reproduction at 1000 ppm for one generation or 250/500 ppm for two generations.
Exposure of progeny to 1400 ppm resulted in reversible effects including lethargy, reduced male fertility, and progeny survival and weight.
A NOEL of at least 500 ppm was established. OSHA PEL and ACGIH TLV for cyclohexanone are 25 ppm (100 mg/m³) with skin absorption notation.
Standard volatile solvent handling precautions apply, including ventilation, avoiding inhalation and skin contact, preventing ingestion, and eye protection.
References
- Cyclohexanol and Cyclohexanone; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a08_217.pub2
- Cyclohexanol and Cyclohexanone. – https://onlinelibrary.wiley.com/doi/full/10.1002/0471238961.0325031206091908.a01
- https://www.sciencedirect.com/science/article/abs/pii/0041008X7990454X
- https://onlinelibrary.wiley.com/doi/10.1002/0471743984.vse2329.pub2
- https://pubchem.ncbi.nlm.nih.gov/compound/Cyclohexanone
