Cumene: Properties, Reactions, Production, Uses and Toxicology
What is cumene?
Cumene, also known as isopropylbenzene or 2-phenylpropane, is an aromatic hydrocarbon with the chemical formula C9H12. It is a colorless, flammable liquid with a characteristic gasoline-like odor.
Cumene occurs as a minor constituent of crude oil and petroleum-derived fuels. It is mainly used as an intermediate in the chemical industry, particularly in the production of phenol and acetone.
Table of Contents
1. Physical properties of cumene
Cumene is a colorless, volatile liquid with a characteristic aromatic odor. It is miscible with many organic solvents, including ethanol, diethyl ether, acetone, benzene, petroleum ether, and carbon tetrachloride. Its solubility in water is low, approximately 61.3 mg/L at 25 °C.
Cumene forms azeotropes with water, aliphatic carboxylic acids, ethylene glycol ethers, cyclohexanol, and cyclohexanone.
Cumene has a variable odor threshold, with reported values ranging from 0.008 to 0.13 ppm. While some studies report human odor perception as low as 0.0032 ppm, others cite values closer to 0.012 ppm. Odor detection is not a reliable method for assessing hazardous exposure to cumene.
The explosion limits for cumene–air mixtures are between 0.8% and 6.0% by volume. This corresponds to a cumene concentration of 40–300 g/m³.
Table 1 lists all the important physical properties of cumene.
| Property | Value |
|---|---|
| CAS number | 98-82-8 |
| Chemical formula | C9H12 |
| Molar mass | 120.2 |
| Freezing point, °C | −96.03 |
| Boiling point, °C | 152.39 |
| Density, g/cm3 (0 °C) | 0.8797 |
| Density, g/cm3 (20 °C) | 0.8633 |
| Density, g/cm3 (40 °C) | 0.8465 |
| Refractive index, n20D | 1.4915 |
| Thermal conductivity at 25 °C, W/(m·K) | 0.124 |
| Viscosity, mPa·s (0 °C) | 1.073 |
| Viscosity, mPa·s (20 °C) | 0.790 |
| Viscosity, mPa·s (40 °C) | 0.610 |
| Surface tension at 20 °C, mN/m | 28.2 |
| Vapor pressure at 35 °C, kPa | 1 |
| Vapor pressure at 100 °C, kPa | 21 |
| Vapor pressure at 120 °C, kPa | 40 |
| Vapor pressure at 140 °C, kPa | 73 |
| Vapor pressure at 180 °C, kPa | 196 |
| Flash point, °C | 33 |
| Autoignition temperature, °C | 425 |
| Flammable limits in air, vol % (lower) | 0.9 |
| Flammable limits in air, vol % (upper) | 6.5 |
| Critical temperature, °C | 358.0 |
| Critical pressure, kPa | 3220 |
| Critical density, g/cm3 | 0.280 |
| Heat of vaporization at boiling point, J/g | 312 |
| Heat of vaporization at 25 °C, J/g | 367 |
| Heat of formation (liquid) at 25 °C, J/mol | −44,150 |
| Free energy (vapor) at 25 °C, J/mol | 137,000 |
| Heat of combustion (gross, water liquid), J/g | 43,370 |
| Heat of combustion (net, water vapor), J/g | 41,170 |
| Heat capacity (liquid) at 25 °C, J/(mol·K) | 197 |
| Heat capacity (ideal vapor) at 25 °C, J/(mol·K) | 153 |
2. Chemical reactions of cumene
The oxidation of cumene to cumene hydroperoxide is the most important industrial reaction of cumene. Other reactions include typical aromatic substitution (nitration, sulfonation, halogenation, alkylation and acylation), side-chain halogenation, hydrogenation, and combustion.
2.1. The cumene process
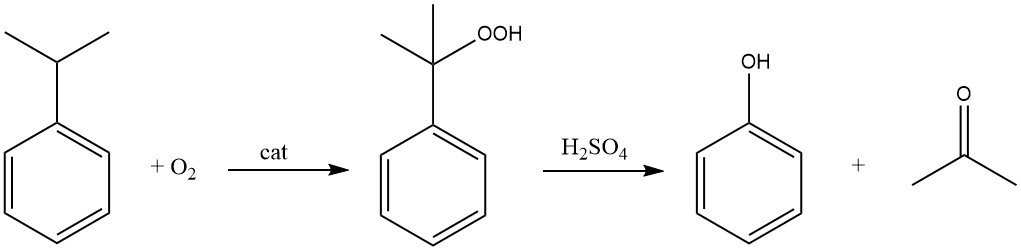
The cumene process, also known as the Hock process, starts with the synthesis of cumene itself, followed by a two-step conversion to phenol and acetone.
The cumene is oxidized by air (oxygen) in the presence of a radical initiator to form cumene hydroperoxide. This reaction targets the tertiary hydrogen atom on the carbon attached to the benzene ring.
Using an acid catalyst like sulfuric acid, the cumene hydroperoxide is then cleaved to produce phenol and acetone.
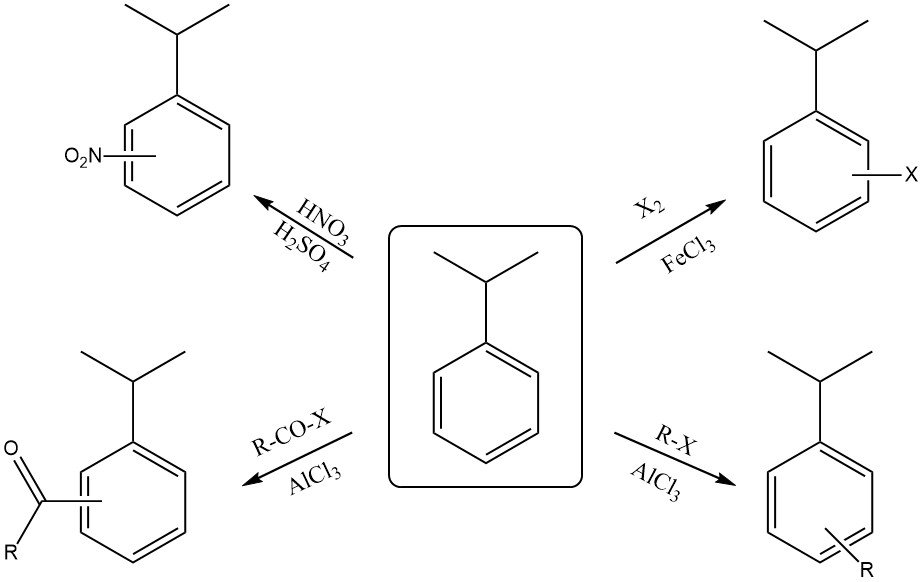
2.2. Reactions on the aromatic ring
Cumene reacts with a mixture of concentrated nitric acid and sulfuric acid to form a mixture of ortho-nitrocumene and para-nitrocumene. The para isomer is typically the major product due to steric hindrance from the bulky isopropyl group.
Halogenation of the ring with reagents like chlorine or bromine in the presence of a Lewis acid catalyst (FeCl3 or FeBr3 ) also produces ortho and para substitution.
Reaction with fuming sulfuric acid yields cumenesulfonic acid.
Cumene can react with an alkyl halide and a Lewis acid catalyst (AlCl3 ) to form alkylcumene.
The reaction of cumene with an acyl halide in the presence of a Lewis acid catalyst (AlCl3 ) yields an ortho- and para-acylcumene.
2.3. Reactions on the alkyl side chain
The benzylic hydrogen (the hydrogen on the carbon directly attached to the benzene ring) is particularly susceptible to radical reactions due to the stability of the resulting benzylic radical.
Cumene can be oxidized by strong oxidizing agents like potassium permanganate or chromic acid. This reaction completely oxidizes the benzylic carbon to a carboxylic acid group to produce benzoic acid and not phenol.
Under high-temperature, UV-light conditions or in the presence of radical initiators, halogens like chlorine or bromine will react exclusively at the benzylic position to replace the hydrogen atom.
Hydrogenation of the aromatic ring gives isopropylcyclohexane using catalysts such as Ni, Pt, or Pd.
Like other hydrocarbons, cumene burns in the presence of oxygen to produce carbon dioxide and water.
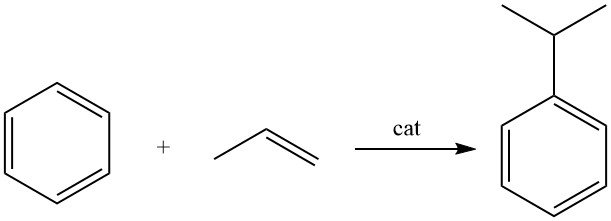
3. Industrial production of cumene
Cumene is produced by the alkylation of benzene with propene in the presence of an acidic catalyst.
Historically, cumene was synthesized in large quantities for use as a component in aviation gasoline during World War II. While it has a high heating value and octane number, it is no longer competitive as a fuel. Its presence in modern gasoline is a minor byproduct of refinery processes like catalytic reforming and steam cracking.
The production of cumene has seen significant advancements with the development of new catalyst technologies, particularly zeolite-based systems, which have replaced older methods that used solid phosphoric acid and aluminum chloride. This technological shift, starting in the mid-1990s, has revolutionized the industry.
In addition to synthetic production, cumene is naturally present in crude oil and refined petroleum products.
3.1. Alkylation and transalkylation reactions
Cumene is produced through the alkylation of benzene with propylene in the liquid phase using an acidic catalyst.
This forward reaction is thermodynamically favorable. However, propylene conversion can be limited by catalyst activity and mass transfer. While n-propylbenzene is thermodynamically more stable than cumene, it’s produced only in minor amounts because the secondary carbon of the propylene molecule is more reactive.
The secondary carbon becomes a carbocation on the catalyst’s acidic site, which then reacts with benzene to form cumene. A high selectivity for cumene over n-propylbenzene, with a ratio of up to 10,000:1, is important since these isomers are not easily separated through distillation.
Further alkylation can also occur, where cumene reacts with more propylene to produce polyisopropylbenzenes, including diisopropylbenzenes, triisopropylbenzenes, and tetraisopropylbenzenes.
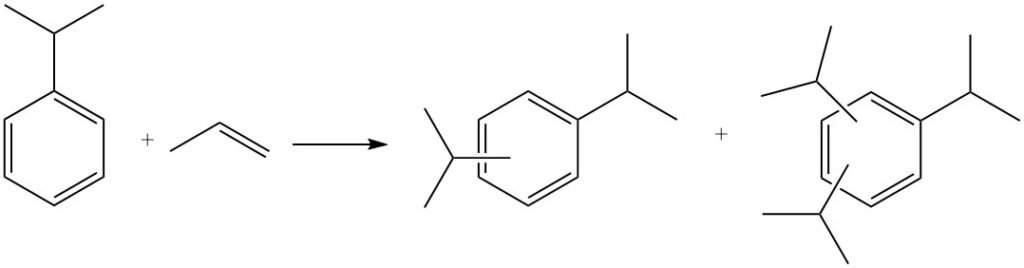
Polyisopropylbenzenes can be reverted to cumene by reacting with benzene in a process called transalkylation.
Alkylation is an exothermic reaction, while transalkylation is essentially isothermal. An excess of benzene is typically used during alkylation to manage the temperature, minimize the formation of polyisopropylbenzenes, and prevent propylene oligomerization.
While a high benzene-to-propylene ratio promotes cumene selectivity, it also increases the cost and energy needed to recover the excess benzene by distillation.
3.2. Production of cumene by solid phosphoric acid (SPA) process
Prior to 1996, the SPA process was the most common method for producing cumene, accounting for over 80% of global production. This process used phosphoric acid supported on alumina as a catalyst.
In this method, which was licensed worldwide by UOP, a mixture of liquid propylene (often a propane-propylene mix) and benzene, along with a small amount of water to maintain catalyst activity, was preheated and fed into a fixed-bed reactor operating at 180–200 °C and about 550 psig.
The typical benzene-to-propylene molar ratio ranged from 5:1 to 8:1. The reactors operated adiabatically, and the heat released by the reaction was partially utilized to vaporize the recycled propane.
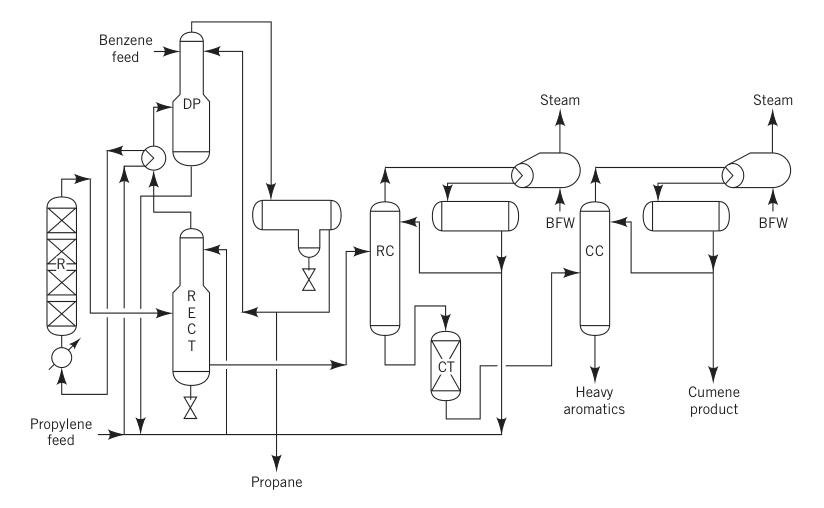
R = reactor; RECT = rectifier; DP= depropanizer; RC = recycle column; CT = clay treater; CC = cumene column; and BFW = boiler feed water.
The SPA process was popular because of its lower initial investment and feedstock flexibility, as it could use refinery-grade propylene and low-purity benzene. Byproducts, such as heavy aromatics, could be used for gasoline blending, which was a key advantage for refineries.
However, this process had a relatively low yield (~5% loss) and produced impurities. Efforts to recover and transalkylate the heavy aromatics were unsuccessful due to high concentrations of impurities like hexylbenzenes.
3.3. Production of cumene by the aluminum chloride process
Another older method for manufacturing cumene used an aluminum chloride complex as the catalyst and operated at temperatures below 100 °C and at low pressure. This process required higher-purity feedstocks and careful dehydration of benzene to maintain catalyst activity and reduce corrosion.
The aluminum chloride process offered the advantage of being able to transalkylate polyisopropylbenzenes to cumene, resulting in higher yields, but it required expensive corrosion-resistant reactors such as one made of Hastelloy or with a glass lining, extensive effluent washing, and created environmental issues associated with catalyst disposal.
3.4. Production of cumene by modern zeolite-based processes
Since the mid-1990s, the cumene industry has rapidly shifted to zeolite-based alkylation technologies. These catalysts eliminated the corrosion and waste disposal problems associated with earlier processes while at the same time provided high yields, high selectivity, and very high product purity.
Modern zeolite catalysts can be regenerated repeatedly, lowering waste and operating costs. They also enable lower benzene-to-propylene feed ratios, which reduces the size of the distillation system and decreases energy consumption.
A notable example is the ExxonMobil/Badger process, which uses a proprietary MCM-22 catalyst. In this liquid-phase process, propylene and benzene are premixed and fed into a fixed-bed alkylation reactor, where propylene is completely consumed. The effluent is then purified through a series of distillation columns.
Excess benzene is recycled, and polyisopropylbenzenes are recovered and sent to a separate transalkylation reactor where they react with benzene to form more cumene.
This process achieves nearly stoichiometric yields and high-purity product. Existing SPA or aluminum chloride plants can be converted to this technology with capacity increases ranging from 30 to more than 100 percent with relatively modest investment.
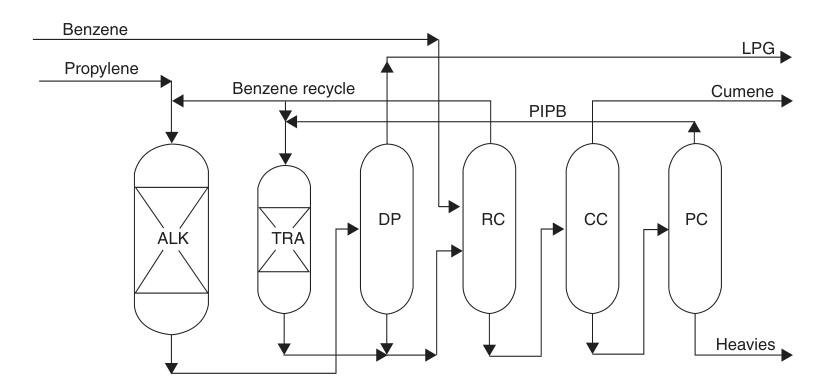
ALK = alkylation reactor; TRA = transalkylation reactor; DP = propanizer; RC = recycle column; CC = cumene column; and PC = polyisopropylbenzenes column.
Other companies, including CDTech, Dow/Kellogg, and UOP, have also developed their own zeolite-based technologies. For example, UOP’s Q-Max process uses a new generation of catalysts that can be regenerated repeatedly, eliminating the need for disposal.
This technology can also expand the capacity of existing plants with minimal capital investment. The shift to these advanced processes has allowed many cumene plants to significantly increase their production capacity.
4. Uses of cumene
Cumene has an octane rating of 109, comparable to that of toluene. During the 1940s this property made it valuable as an additive for aviation gasoline.
Cumene is now used almost entirely (95% of total production) as an intermediate for the production of phenol and acetone. In the presence of oxygen, cumene undergoes oxidation to cumene hydroperoxide, which upon acid-catalyzed cleavage yields phenol and acetone.
Global production of cumene reached about 18 million tons in 2020 and was projected to increase, mainly driven by the growing demand for bisphenol A in polycarbonate and epoxy resin manufacture.
Minor by-products formed during the cumene oxidation process include α-methylstyrene, acetophenone, cumyl alcohols, and diisopropylbenzene.
α-Methylstyrene is used in the production of acrylonitrile–butadiene–styrene (ABS) resins, p-cumylphenol, and other intermediates.
Cumene hydroperoxide is also used as a radical initiator in the copolymerization of styrene with butadiene and acrylates and in the cross-linking of unsaturated polyester resins. The reaction of cumene hydroperoxide with α-methylstyrene gives dicumyl peroxide, an initiator for radical cross-linking of polyolefins.
Hydrogenation of cumene yields isopropylcyclohexane (hydrocumene), which is a high-boiling (154.5 °C), low-freezing (–90 °C) cycloaliphatic solvent.
Cumene is also used in smaller quantities as a thinner for paints, enamels, and lacquers, and as a solvent for fats and resins, sometimes replacing benzene in industrial applications.
According to the Consumer Product Information Database (2022), cumene is found in more than one hundred consumer and household products, including pesticides and cleaning agents.
5. Toxicology of cumene
Cumene exposure occurs primarily through inhalation of contaminated air in occupational settings and from the evaporation of petroleum products. Additional sources include cigarette smoke, contaminated food and water, and accidental ingestion or dermal absorption.
Inhalation is the most significant route, as cumene is readily absorbed through the lungs, metabolized in the liver to water-soluble compounds, and efficiently excreted, mainly in urine, without evidence of long-term accumulation.
Human toxicokinetic studies confirm that cumene and its metabolite 2-phenyl-2-propanol are eliminated within 40 hours of exposure.
Acute exposure to high concentrations of cumene induces central nervous system depression, manifested as headache, dizziness, narcosis, nausea, and loss of consciousness at levels well above occupational exposure limits.
Cumene is also a primary irritant to the skin, eyes, and respiratory tract. Repeated or prolonged contact with the skin may cause dermatitis and rashes, while ingestion carries a high risk of aspiration pneumonitis.
In animal studies, short-term inhalation at concentrations above 1000 ppm induced reversible neurotoxic effects, whereas exposure to very high doses led to severe central nervous system toxicity and death.
The oral LD50 in rats is approximately 1400 mg/kg, and inhalation LC50 values range between 2000 and 8000 ppm depending on species.
| Exposure route | Symptoms | First aid action |
|---|---|---|
| Inhaling and smelling |
Dizziness Incoordination Drowsiness Headache |
Remove to fresh air and rest Get medical attention if necessary |
| Skin contact |
Dry skin Rashes Itchiness Red skin |
Remove the contaminated clothing immediately and wash the skin with water Some references suggest washing the skin with water and soap after rinsing If irritation persists after washing, consult a physician |
| Eye contact |
Eye irritation Redness |
Immediately rinse the eyes with large quantities of water, occasionally lifting the lower and upper lids, if the chemical contacts the eyes. Take immediate medical care |
| Swallowing and ingestion |
Dizziness Incoordination Drowsiness Headache |
There is a risk of aspiration Mouthwash and rinsing are recommended. Avoid inducing vomiting Refer to a medical professional instantly |
Chronic human data are limited, but animal studies indicate mild hematological and organ effects following subchronic inhalation exposure, such as passive congestion of the lungs, liver, kidneys, and spleen. No consistent immunotoxic effects have been reported.
Evidence for reproductive and developmental toxicity is weak, with studies suggesting rapid metabolism and excretion without significant effects on fertility or offspring development.
Genotoxicity testing has produced mostly negative results in bacterial, in vitro, and in vivo systems, though some high-dose animal studies reported weak cytogenetic effects.
Carcinogenicity data in humans are inadequate, but long-term inhalation studies in rodents demonstrated increased incidences of liver, kidney, and lung tumors, leading the U.S. National Toxicology Program to classify cumene as “reasonably anticipated to be a human carcinogen.”
The International Agency for Research on Cancer (IARC) has also listed cumene as a possible human carcinogen (Group 2B).
The Occupational Safety and Health Administration (OSHA) and the American Conference of Governmental Industrial Hygienists (ACGIH) have established an 8-h time-weighted average permissible exposure limit and threshold limit value of 50 ppm (245 mg/m³).
While acute effects are generally reversible, the slow induction and elimination of its central nervous system depressant action suggest potential cumulative risks.
References
1. Hwang, S.Y. and Chen, S.S. (2010). Cumene. In Kirk-Othmer Encyclopedia of Chemical Technology, (Ed.). https://doi.org/10.1002/0471238961.0321130519030821.a01.pub3
2. Abdolmaleki, G.; Bayrami, Z. Cumene. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press, 2024; pp 337–343. DOI: 10.1016/B978-0-12-824315-2.00295-5
3. Dimian, A.C. and Bildea, C.S. (2008). Alkylation of Benzene by Propylene to Cumene. In Chemical Process Design (eds A.C. Dimian and C.S. Bildea). https://doi.org/10.1002/9783527621583.ch6
4. Gollapudi, B. B.; Williams, A. L.; Bus, J. S. “A review of the genotoxicity of the industrial chemical cumene.” Mutat. Res./Rev. Mutat. Res., 2021, 787, 108364. DOI: 10.1016/j.mrrev.2021.108364
5. Schmidt, R., Griesbaum, K., Behr, A., Biedenkapp, D., Voges, H.-W., Garbe, D., Paetz, C., Collin, G., Mayer, D. and Höke, H. (2014). Hydrocarbons. In Ullmann’s Encyclopedia of Industrial Chemistry, (Ed.). https://doi.org/10.1002/14356007.a13_227.pub3
