Cinnamic Acid: Properties, Production and Uses
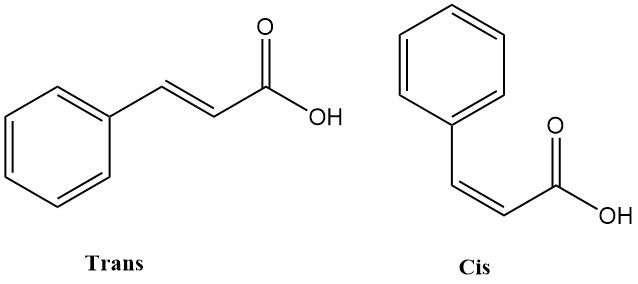
Cinnamic acid, also known as 3-phenylpropenoic acid, is an organic compound with the chemical formula C6H5CH=CHCOOH that is found in nature and commercially available. It exists in two isomeric forms: cis and trans. The trans isomer is more stable and is the predominant form found naturally and commercially.
In 1780, cinnamic acid was isolated as crystals from cinnamon oil by Trommsdorf, who mistook it for benzoic acid. Later, in 1835, Dumas and Péligot identified it. Bertagnini successfully synthesized cinnamic acid from benzaldehyde and acetyl chloride in 1856.
Table of Contents
1. Physical Properties of Cinnamic Acid
The physical properties of trans-cinnamic acid are as follows:
- CAS Number: 140-10-3
- Molecular weight: 148.16 g/mol
- Physical Appearance: Colorless crystals with a faint balsamic odor
- Melting Point: 133 °C
- Boiling Point: 300 °C (101.3 kPa) and 173 °C (13.3 kPa)
- Density: 1.2475 g/cm³ at 4 °C and 1.0270 g/cm³ at 180 °C
- pKa at 25 °C: 4.46
- Heat of Combustion: 30.5 kJ/g
- Solubility: Slightly soluble in water, highly soluble in polar organic solvents like ethanol, methanol, chloroform, and acetone.
The cis-cinnamic acid [102-94-3] exists in three crystalline forms: cis-allocinnamic acid (mp 68 °C) and two cis-isocinnamic acids (mp 58 °C and 42 °C).
The lower-melting cis-isocinnamic acid is highly unstable and readily converts to cis-allocinnamic acid. Interconversion between the three forms is possible by inoculating their melts.
2. Chemical Reaction of Cinnamic Acid
Cinnamic acid exhibits reactivity characteristic of both its carboxyl group and the olefinic double bond.
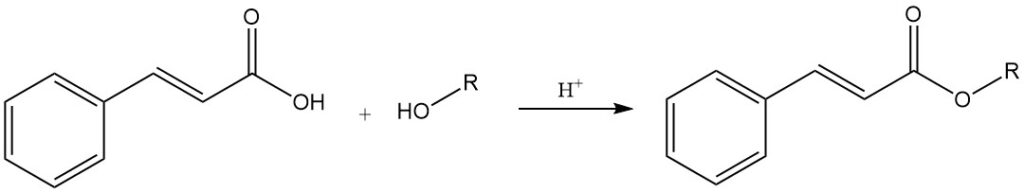
Cinnamic acid reacts with alcohols to form cinnamate esters, some of which possess significant flavoring and fragrance properties. It reacts with inorganic acid chlorides (thionyl chloride and phosphorus chlorides) to yield cinnamoyl chloride.
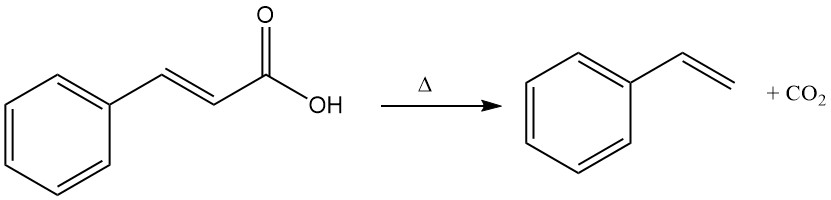
Cinnamic acid undergoes thermal decomposition (decarboxylation) to form styrene and carbon dioxide.
Benzaldehyde can be produced by cleaving the double bond with oxidizing agents or by heating with alkali.
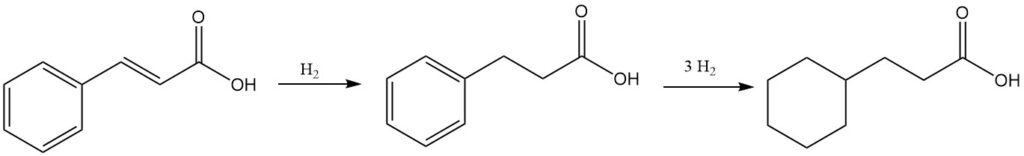
Cinnamic acid can be hydrogenated to give either 3-phenylpropionic acid or 3-cyclohexylpropionic acid, depending on the reaction conditions.
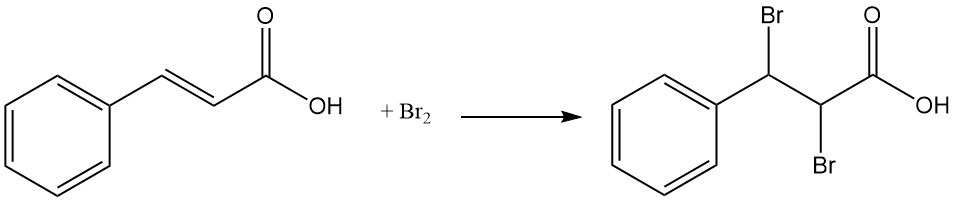
Under sunlight, cinnamic acid forms dimers, including truxinic acid and α-truxillic acid.
Substitution reactions on the benzene ring of cinnamic acid are rarely employed to produce new derivatives.
3. Occurrence in Nature
Cinnamic acid, predominantly in its trans form, is naturally found in both free and esterified forms (cinnamates) within various plant materials.
The free form is found in:
- Cassia oil
- Extract oil from Populus balsamifera
- Essential oils from leaves and peels of Citrus bigaradia
Different cinnamate esters are found in plant materials, including methyl cinnamate found in the oils of Alpinia species and Ocimum canum varieties and benzyl, cinnamyl, and hydrocinnamyl cinnamates, which are present in Peru, Tolu, and storax balsam oils. Siam and Sumatra benzoin resins also contain cinnamates.
4. Production of Cinnamic Acid
Commercial production of cinnamic acid predominantly favors the trans isomer through various established processes:
4.1. Production of Cinnamic Acid by Perkin Reaction
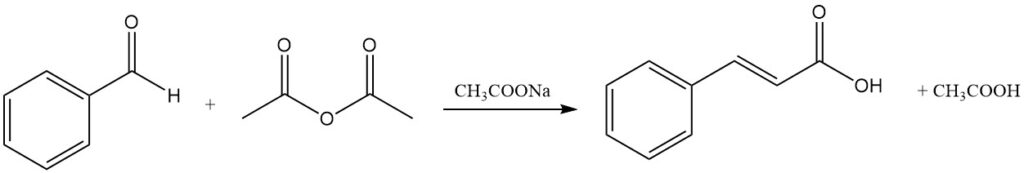
The Perkin reaction is the traditional commercial method for producing cinnamic acid by the condensation of benzaldehyde with acetic anhydride in the presence of a catalyst, typically sodium acetate. Other catalysts, like potassium acetate, tertiary amines, potassium phosphate, and trimethyl borate, can also be used.
4.2. Production of Cinnamic Acid by Claisen Condensation
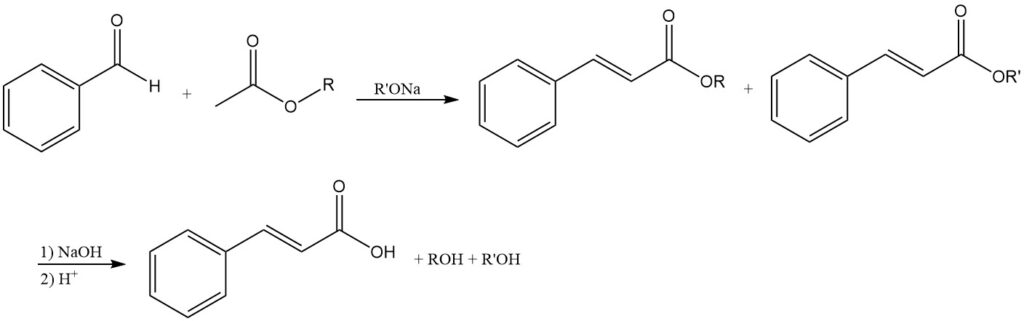
The condensation of benzaldehyde with acetic acid esters using alkali alcoholates produces cinnamic acid esters as the initial products, which, after saponification, yield cinnamic acid.
4.3. Production of Cinnamic Acid from Benzal Chloride
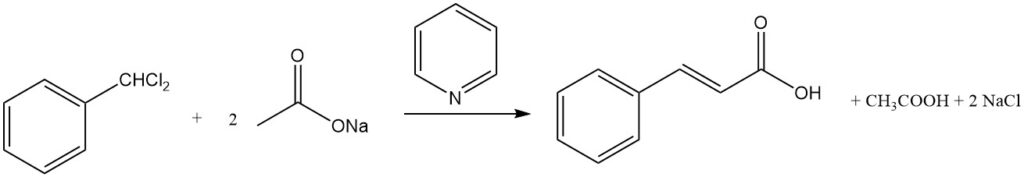
Cinnamic acid can be synthesized in high yields by the reaction of benzal chloride and alkali acetate in an alkaline medium. The presence of amines like pyridine can further enhance the yield to over 80%.
4.4. Production of Cinnamic Acid by Oxidation of Cinnamaldehyde
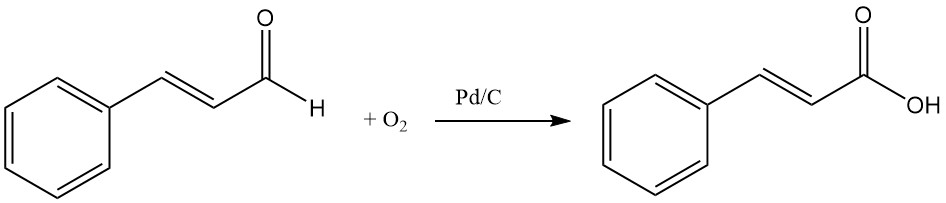
Cinnamic acid can be produced by the oxidation of cinnamaldehyde using oxygen and catalysts such as silver or palladium on charcoal.
4.5. Preparation of Cinnamic Acid from its Esters
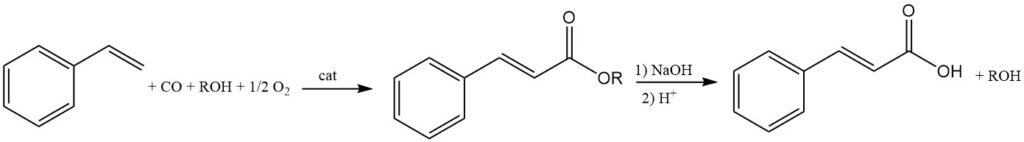
Cinnamic acid esters are formed by the reaction of styrene, alcohols, carbon monoxide, and oxygen using platinum or palladium catalysts. The desired cinnamic acid can then be obtained from the hydrolysis of these esters.
5. Uses of Cinnamic Acid
Cinnamic acid is used as a precursor to the production of artificial sweeteners (aspartame) and flavoring agents in foods and beverages and has potential applications in cosmetics and pharmaceuticals due to its antimicrobial and anti-inflammatory properties.
Cinnamic acid esters, derived from cinnamic acid, are widely used in perfumes and cosmetics for their pleasant aromas.
Cinnamic acid is used as an intermediate in the enzymatic production of L-phenylalanine, a key component of peptide sweeteners (Aspartame).
Sodium cinnamate effectively inhibits corrosion and finds use in various applications.
Cinnamic acid acts as a corrosion inhibitor during zinc electroplating (without cyanide) and the removal of scale from zinc surfaces and in aerosol cans.
Cinnamic acid is used as a low-toxicity heat stabilizer for PVC, as a cross-linking agent for various polymers, including polyurethanes and dimethyl terephthalate-ethylene glycol copolymers, and as a fire retardant for polycaprolactam.
It is also used in the production of laundry-resistant polyurethane adhesives for polyester fibers and for improving the storage stability of drying-oil-modified alkyd resin coatings.
6. Toxicology of Cinnamic Acid
Acute Toxicity
- Oral (rats): LD50 = 2.5 g/kg (relatively low, indicating moderate oral toxicity)
- Dermal (rabbits): LD50 > 5 g/kg (relatively high, indicating low dermal toxicity)
Skin Irritation
Neat cinnamic acid causes slight irritation to intact or abraded rabbit skin after 24-hour exposure.
Skin Sensitization
A 4% solution of cinnamic acid in petrolatum did not cause sensitization in human subjects.
Reference
- Cinnamic Acid; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a07_099
