Chemical Reactions of Aliphatic Amines
Aliphatic amines, characterized by the presence of an amino functional group attached to a carbon chain, exhibit diverse chemical reactions owing to the electron lone pair on the nitrogen atom and the tendency of hydrogen atoms bonded to nitrogen to be substituted by other groups.
Table of Contents
1. Amine Salts Formation
One important aspect of aliphatic amines is their ability to form salts when they react with acids. Due to the alkyl substituents they carry, aliphatic amines are stronger bases than ammonia. Consequently, they readily react with acids to form water-soluble salts that are insoluble in organic solvents.

This property makes amines excellent acid acceptors and useful solvents in gas scrubbing and extraction processes (e.g., in the synthesis of semisynthetic penicillins).
Notably, tertiary amines such as trimethylamine, tributylamine, and ethyldiisopropylamine are widely employed as proton acceptors and catalysts in organic synthesis because they can be removed from the organic mixture after reaction.
2. Conversion to Carboxamides
The reaction between amines and carboxylic acids, as well as their esters, chlorides, and anhydrides, leads to the production of substituted carboxamides:

This transformation generally results in high yields, particularly when employing carboxylic acid chlorides, which is a highly exothermic reaction. When reacting with carboxylic acids, the reaction often ceases at the formation of the initial ammonium salt.
This reaction type finds significant industrial application, such as in the synthesis of various herbicides possessing an acid amide structure.
When an alkylamine reacts with a lactone through this process, a lactam is generated. A notable example of this reaction is the formation of 1-ethyl-2-pyrrolidone from the combination of ethylamine and γ-butyrolactone.
3. Conversion of Amines to Sulfonamides
The reaction of aliphatic amines with benzenesulfonyl chloride is used for distinguishing between primary, secondary, and tertiary amines, commonly known as the Hinsberg test. Additionally, this reaction can be employed for the preparative separation of these amine types.
In this test, primary amines yield alkali-soluble N-alkyl benzenesulfonamides, while secondary amines produce alkali-insoluble N,N-dialkyl benzenesulfonamides. Tertiary amines, on the other hand, do not exhibit any reactivity under these specific conditions.
It should be noted that limitations may arise with long-chain primary amines, as they can remain alkali-insoluble despite the presence of acidic hydrogen. Tertiary amines can also undergo quaternization with sulfonyl chloride, leading to altered reaction outcomes.

Sulfonamides once played a vital role as pharmaceuticals in the treatment of bacterial infections, saving countless lives during the Second World War. However, they have since been replaced by safer alternatives such as penicillin and other antibiotics.
4. Reaction of Amines with Carbonyl Compounds
Under specific reaction conditions and with suitable compounds, carbonyl compounds can undergo reactions with amines, resulting in the formation of imines (Schiff bases) (2) or enamines (3). These products can further undergo hydrogenation to yield more highly alkylated amines. This reaction holds significant importance as a method for synthesizing higher amines.
It is worth noting that the intermediate hemiaminals (1) formed during the reaction are usually not isolable. Aldehydes generally react faster than ketones. Secondary amines exclusively form enamines, while tertiary amines do not yield either of the two products.

A reaction involving an amine, an aldehyde, and a compound possessing an activated hydrogen atom, such as a ketone, leads to the formation of a Mannich base.
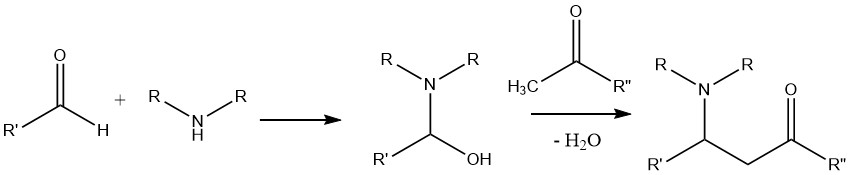
In this reaction, the condensation product formed between the aldehyde and the amine is subjected to attack by the α-C—H acidic compound, which originates from the ketone.
5. Reaction of Amines with Carbon Dioxide and Carbon Disulfide
The carbamic acid or dithiocarbamic acid generated during this reaction exhibits instability, yet it can be isolated in the form of a salt or an ester.

Dithiocarbamates derived from diverse amines hold significant importance as vulcanization accelerators in the rubber industry.
6. Reaction of Amines with Epoxides
When primary amines undergo a reaction with epoxides, a mixture of mono- and dioxyalkylated derivatives is formed. On the other hand, secondary amines exclusively yield monooxyalkylated compounds, while tertiary amines yield quaternary ammonium compounds.
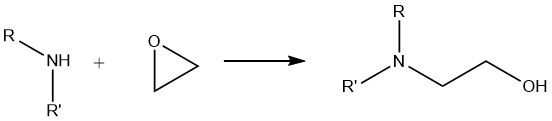
This alkoxylation reaction is very important in the industrial application of aliphatic amines. It is widely utilized in the synthesis of flocculants, surface coating resins, drug intermediates, and products employed in gas scrubbing processes such as ethanolamine and propanolamine.
7. Alkylation of Amines
The reaction of amines with alkyl halides and dialkyl sulfates ultimately leads to the formation of quaternary ammonium compounds, which find extensive application in preparative pharmaceutical chemistry as well as in the synthesis of anticorrosion agents and biocides.
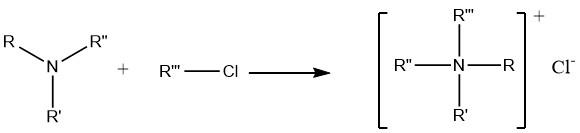
However, the reaction between alkyl halides and ammonia is not particularly useful for the preparation of primary or secondary amines. This is due to the stronger basicity of primary and secondary amines, causing them to preferentially attack the halides rather than undergo substitution reactions.
However, tertiary amines can still be prepared through these reactions. In laboratory-scale syntheses, primary amines can be obtained using hexamethylenetetramine, while secondary amines can be synthesized using cyanamide.
8. Formation of Isocyanates and Ureas
The reaction between phosgene and primary amines initially yields the corresponding carbonyl chloride. Subsequent cleavage of hydrogen chloride leads to the formation of alkyl isocyanate. The excess amine then reacts with the isocyanate, resulting in the formation of ureas.
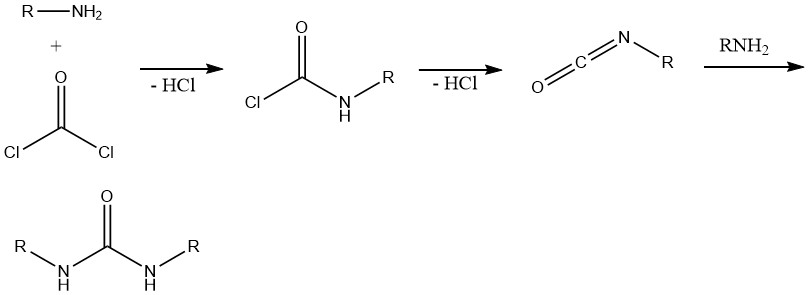
This reaction is important in the synthesis of various herbicides containing urea, carbamate, or thiocarbamate structures and in polyfunctional amine systems, mainly polyurethane chemistry.
Similarly, secondary amines react with phosgene via an analogous intermediate chloride, leading to the formation of N,N’-tetraalkylureas.
9. Reaction of Amines with Acrylonitrile
The industrial-scale addition of a primary or secondary amine to acrylonitrile, known as the 1,2-Michael addition, is widely used for the synthesis of higher diamines and polyamines. This reaction is favored because the nitrile functionality can be easily hydrogenated into the corresponding amine.

10. Formation of Isonitriles
Under basic conditions, primary amines undergo a reaction with trichloromethane to produce isonitriles.
The strong and unpleasant odor of isonitriles allows this reaction to be utilized as a test for the presence of primary amines. Additionally, for lower amines, this reaction can also be employed as a synthetic method.
An alternative approach involves the use of N-alkylformamides as starting materials for the synthesis of isonitriles.
11. Oxidation of Amines
Free amines are sensitive to oxidation, contrary to their salts, resulting in the formation of various products depending on the oxidizing agent and the type of amine involved.
Tertiary amines, when oxidized with hydrogen peroxide, form amine oxides. On the other hand, primary and secondary amines, upon oxidation, yield corresponding hydroxylamines or aldoximes by subsequent reactions with the formed compounds.

The use of nitrous acid for oxidation serves as a means to differentiate between primary, secondary, and tertiary amines. Primary amines undergo diazotization, followed by the elimination of gaseous nitrogen, and eventually form alcohols through the reaction of the intermediate carbenium ion with water.
Secondary amines react to produce yellow N-nitrosoamines. Typically, tertiary amines do not exhibit reactivity under these conditions.
The formation of nitrosamines can be a significant concern, particularly with secondary amines, as they are highly mutagenic. While they are not typically formed during normal usage, trace amounts can be formed upon contact with nitrites or nitrous oxides, resulting in handling issues. Air contamination due to secondary amines is thus regarded as problematic.
12. Dealkylation of Amines
After converting tertiary amines into basic quaternary ammonium salts, they undergo dealkylation upon heating. In cases where only methyl groups are present, they are eliminated as methanol or dimethyl ether. However, when higher alkyl groups are present, they yield alkenes, as exemplified in the reaction:

This reaction, commonly referred to as the Hofmann degradation, is not frequently employed in synthetic applications. However, it played a significant role in the past in elucidating the structural characteristics of unknown amines.
Reference
- Amines, Aliphatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_001.pub2
