Butyric Acid: Properties, Reactions, Production and Uses
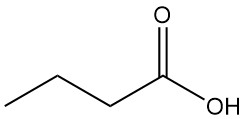
What is Butyric Acid?
Butyric acid, also known as n-butanoic acid, is a short-chain volatile fatty acid with the chemical formula C4H8O2. It is a colorless, oily liquid with an unpleasant, rancid odor that is missible with water and most organic solvents.
Butyric acid was discovered in 1869 by Lieben and Rossi. In Latin, butyric acid means the acid of butter, as it was first discovered in rancid butter (butyric acid is hydrolyzed from the glyceride and causes a very unpleasant odor).
Butyric acid is a short-chain saturated fatty acid found in the form of esters in animal fats and plant oils and is naturally produced by anaerobic bacteria.
Table of Contents
1. Physical Properties of Butyric Acid
Butyric acid is a carboxylic acid that forms an oily, colorless liquid that is soluble in water, ethanol, and ether and slightly soluble in carbon tetrachloride.
Table 1 summarizes the physical properties of butyric acid.
| Property | Value |
|---|---|
| CAS number | [107-92-6] |
| Chemical formula | C4H8O2 |
| Molecular weight | 88.1 g/mol |
| Melting point | -7.9 °C |
| Boiling point | 163.5 °C |
| Density | 0.958 g/cm3 |
| pKa | 4.82 at 25 °C |
| Viscosity at 25 °C | 1.426 mPa.s |
| Refractive Index | 1.3991 at 20 °C |
| Critical temperature | 342.05 °C |
| Critical pressure | 4.06 MPa |
| Vapor pressure | 1.65 mmHg |
| Flash point | 72 °C |
| Autoignition temperature | 443 °C |
2. Chemical Reactions of Butyric Acid
The carboxylic group of butyric acid undergoes different types of reactions, mainly deprotonation, reduction, and nucleophilic substitution. Other reactions take place at the α-position of the carboxylic group due to the presence of an acidic hydrogen.
Alkali metal salts of butyric acid are readily obtained by neutralization using a base (e.g., sodium hydroxide). Ammonium butyrates are obtained by reaction with ammonia or amines.
n-Butanol can be prepared by reduction of the carboxyl group using reducing agents such as lithium aluminum hydride or via catalytic hydrogenation of the butyric acid or its esters.
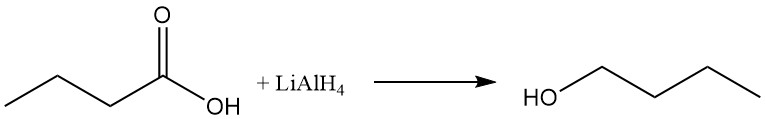
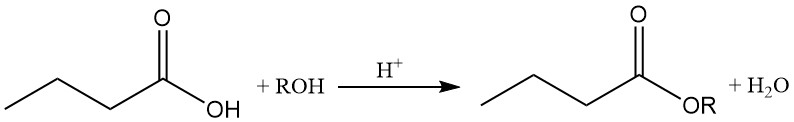
Butyric acid esters are produced by an acid-catalyzed reaction between butyric acid and alcohols or olefins. Butyrates are important intermediaries in different industries.
Butyric amides can be synthesized directly by reacting butyric acid with ammonia or amines. As the ammonium butyrate is formed first, heating is required to remove water and drive the reaction.
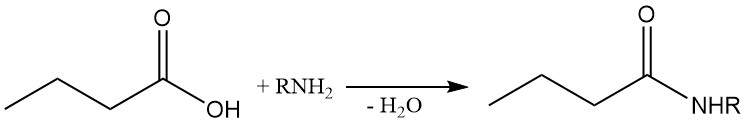
Butyric anhydride is prepared by dehydration of butyric acid using a dehydrating agent (e.g., phosphorus pentoxide) or, preferably, by the reaction of butyryl chloride with butyric acid or its salt.
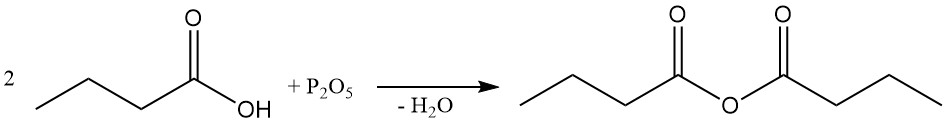
Butyryl halides can be obtained from butyric acid using halogenating compounds like thionyl halides, phosphorus halides, or phosphorus oxyhalides. The highly reactive, hydrolysis-sensitive butyryl halides are a valuable class of synthetic intermediates.
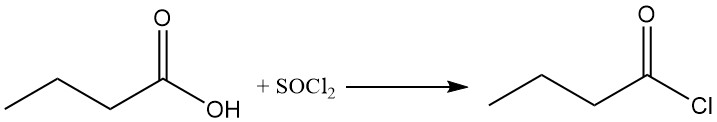
The oxidation of butyric acid with hydrogen peroxide yields acetone, carbon dioxide, and water.
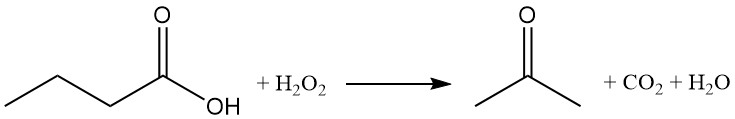
Under certain conditions, butyric acid can undergo decarboxylation.
Butyric acid having at least one hydrogen at the carbon next to the carboxyl function can also be deprotonated in that α-position, but this tendency is by far not as pronounced as for aldehydes and ketones. Two equivalents of a strong, nonnucleophilic base are necessary.
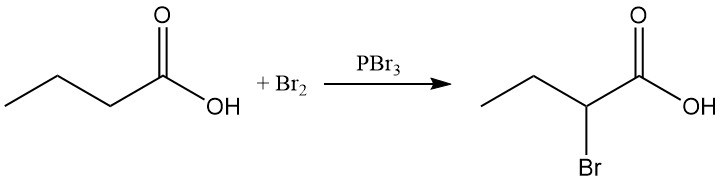
In the Hell-Volhard-Zelinsky halogenation, using bromine and phosphorus tribromide, 2-bromobutyric acid can be synthesized.
3. Production of Butyric Acid
3.1. Chemical Production of Butyric Acid
Commercially, n-butyric acid is produced by liquid-phase oxidation of n-butanal with oxygen. This continuous process yields a colorless liquid, which is purified by rectification.
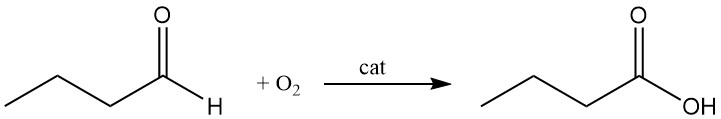
The hydroformylation process (Oxo process) of propylene is the primary production method for n-butanal. It involves propylene hydroformylation using syngas (primarily hydrogen and carbon monoxide) at high temperatures (100–180 °C) and pressures between 20 and 30 MPa in the presence of a metallic catalyst.
The LP Oxo (triphenylphosphine-modified rhodium oxo reaction) process yields up to 90% crude butyric acid. However, the Oxo process generates both butyraldehyde and isobutyraldehyde, which reduces butyric acid selectivity. Additionally, the process produces environmentally harmful byproducts, such as spent metallic catalysts and toxic compounds.
To overcome these issues, a one-step oxidation of butyraldehyde to butyric acid using molecular oxygen and heterogeneous catalysts has been developed. This process achieves higher butyric acid yields exceeding 99.5% and selectivity greater than 95% compared to the Oxo process.
Alkali metal and cobalt salts are highly active heterogeneous catalysts used in this process.
In a patent by Huntsman Petrochemical Corporation, butyric acid can be produced by catalytic hydrogenation of maleic anhydride with a selectivity of 35% for butyric acid.
Major manufacturers of butyric acid include Oxea, Eastman, and Perstorp.
3.2. Biosynthesis of Butyric Acid
Butyric acid biosynthesis occurs under anaerobic conditions. Acetyl-CoA, derived from fatty acid degradation or pyruvate via pyruvate-ferredoxin oxidoreductase, serves as a precursor. Acetyl-CoA is transformed into butyryl-CoA, subsequently yielding butyric acid.
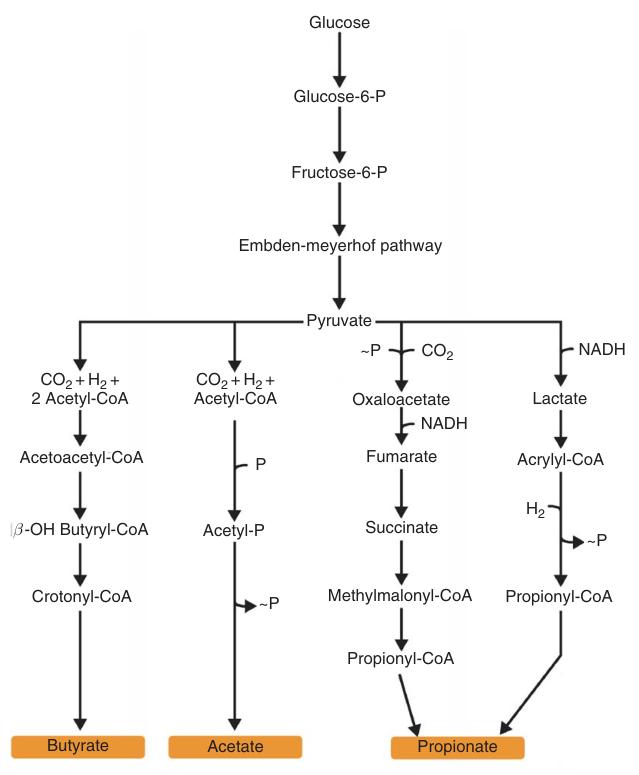
Anaerobic fermentation of glucose to butyric acid generates three ATP molecules, exceeding the ATP yield of lactic acid/ethanol fermentation (two ATP) and approaching that of acetic acid fermentation (four ATP).
Butyric acid is a fermentation end product produced by obligate anaerobic bacteria, with Clostridium butyricum as the primary organism. Other butyric acid-producing bacteria include Clostridium, Butyribacterium, Sarcina, Megasphera, Fusobacterium, Peptococcus, Butyrivibrio, Pseudobutyrivibrio, and Eubacterium species.
Optimal growth conditions encompass 35–37 °C, a CO2/N2 atmosphere, and a pH range of 4.5 to 7.0. Hexoses, pentoses, and polysaccharides can be used as raw materials.
Butyric acid production is optimized by controlling nutrient availability. Limiting glucose and essential elements like phosphate and nitrogen while using slow growth conditions favors butyrate formation over other products. To enhance efficiency, batch, fed-batch, or continuous fermentation methods can be combined with cell retention techniques.
The accumulation of butyric acid during the fermentation process can reduce the production process. To prevent this, the concentration of butyric acid in the fermentation broth is reduced by distillation, evaporation, permeate electrodialysis, adsorption, extraction, or pertraction.
4. Uses of Butyric Acid
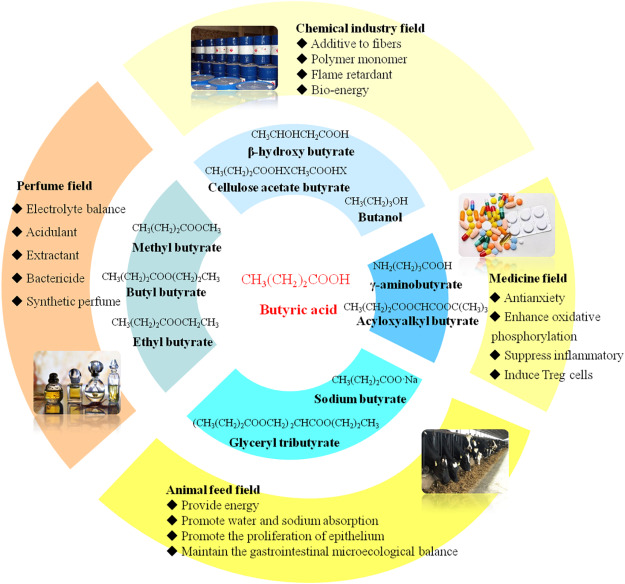
Butyric acid is primarily used in the production of cellulose acetate butyrate, a polymer employed in the manufacture of plastics, coatings, and textiles.
In the food industry, butyric acid and its derivatives are used as flavoring agents, contributing to the characteristic taste of butter, cheese, and confectionery. Additionally, it is utilized as a food preservative due to its antimicrobial properties.
Butyric acid esters are used in perfumes, and some serve as the bases of artificial flavoring ingredients in certain liquors, soda-water syrups, and candies.
Butyric acid is used in formulations for preparing fishing bait and wildlife repellents due to its powerful odor.
Butyric acid is used in pharmaceuticals as a histamine antagonist. Its derivatives have demonstrated potential as anticancer agents by inducing cellular differentiation. Butyric acid prodrugs have been suggested for the treatment of neoplastic diseases and β-globin disorders.
It is also used as an intermediate in the production of butyroperoxides, herbicides, emulsifiers, disinfectants, and surfactants.
In the agricultural industry, butyric acid is employed as a feed additive and diet supplement for pigs, cattle, and poultry farming. Furthermore, it has shown efficacy in preventing fungal growth in high-moisture grains.
Recent research has explored the potential of butyric acid as a precursor for biofuel production. Its conversion to butanol, a higher-energy alcohol, is an active area of investigation.
5. Toxicology of Butyric Acid
While butyric acid is not used in consumer products, exposure to the general population occurs primarily consuming foods containing it, such as animal milk fats, essential oils, vegetable oils, animal fluids, fermented products and in certain fruits.
Butyric acid is metabolized rapidly in the liver, primarily to acetic acid and ketone bodies (acetone, acetoacetate, and β-hydroxybutyrate). The elimination of butyric acid follows a biphasic pattern in humans. An initial rapid phase with a half-life of 0.5 minutes is followed by a slower elimination phase with a half-life of 13.7 minutes.
Acute toxicity
Butyric acid is classified as non-toxic based on LD50 values in various animal models.
- Oral LD50 in rats: 2940–8790 mg/kg
- Intraperitoneal or subcutaneous LD50 in mice: 3180 mg/kg
- Inhalation LC50 in rabbits: 440 mg/l
Butyric acid is a moderate irritant to skin and eyes. Severe eye irritation and corneal burns were observed in rabbits. Increased lethargy, dyspnea, bronchial and capillary dilation, and emphysema were noted in rabbits exposed to butyric acid aerosol (40 mg/l).
High intravenous doses of butyric acid (sodium salt) cause temporary CNS depression in rabbits and dogs. Increased plasma insulin concentration was observed in lambs following intravenous butyric acid administration.
Acute exposure in humans to butyric acid primarily results in irritation or burns to the skin, eyes, and respiratory tract.
In humans, inhalation symptoms include a sore throat, cough, burning sensation, shortness of breath, and labored breathing. Dermal contact leads to pain, redness, blisters, and skin burns. Eye contact causes pain, redness, severe burns, and potential vision loss.
Ingestion results in a burning sensation, abdominal pain, shock, and potential collapse.
Chronic toxicity
In animal studies, repeated exposure to moderate to high doses of n-butyl acetate and n-butanol (metabolites of butyric acid) is generally well-tolerated in animals. However, high doses of butyric acid in the diet led to weight loss and gastric tissue abnormalities in rats.
In human studies, butyric acid is considered a GRAS (Generally Recognized As Safe) food additive. Chronic exposure can occur through endogenous production and dietary intake. While it’s an essential metabolite, prolonged exposure can damage lungs, nervous system, and mucous membranes.
Immunotoxicity
Prolonged exposure of mice, rats, and rabbits to low atmospheric concentrations of butyric acid (0.1–0.2 mg/l) resulted in a significant increase in circulating lymphocytes and neutrophils.
This immune response is primarily attributed to the irritant nature of butyric acid rather than a specific immune system targeting.
Reproductive toxicity
Studies on the reproductive toxicity of butyric acid are primarily based on its metabolites, n-butanol and n-butyl acetate.
Inhalation studies on n-butanol and n-butyl acetate showed no adverse effects on reproductive parameters up to certain concentrations:
- Female reproductive toxicity: 6000 ppm for n-butanol and 1500 ppm for n-butyl acetate.
- Male reproductive toxicity: 3000 ppm for n-butyl acetate.
Developmental toxicity
Direct administration of butyric acid to pregnant rats resulted in high maternal mortality and reduced body weight gain at high doses (100 and 133 mg/kg/day). Despite maternal toxicity, no adverse effects were observed in the offspring.
Butyric acid induces malformations (microcephaly, eye defects, edema, and gut defects) in frog embryos at a concentration of 400 mg/l.
Genotoxicity and Carcinogenicity
Butyric acid is not genotoxic or carcinogenic.
References
- Carboxylic Acids, Aliphatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a05_235.pub2
- https://www.sciencedirect.com/science/article/abs/pii/B9780123864543005911
- https://www.sciencedirect.com/science/article/abs/pii/B9780128096338130833
- Process of Making Butyric Acid. – https://patents.justia.com/patent/20080009652
- https://www.sciencedirect.com/science/article/abs/pii/S0734975018301629
- https://onlinelibrary.wiley.com/doi/10.1100/2012/471417
- Fermentation process for producing butyric acid. – https://patents.google.com/patent/US2181310A/en
