Benzene: Properties, Reactions and Uses
Benzene is a single-ring aromatic compound with the formula C6H6. It is a colorless, flammable liquid with a sweet odor. Benzene is thermally stable and chemically reactive, making it a valuable precursor to many other chemicals.
Benzene is primarily used to produce styrene, phenol, and cyclohexane, which are used in the manufacture of plastics, resins, and other products. It is also used as a solvent, but its use has declined due to its high toxicity. Benzene is also a component of gasoline, where it is used to boost octane rating.
Benzene was first isolated by Michael Faraday in 1825, and commercial production processes were developed in the mid-19th century. Today, benzene is primarily obtained from petroleum.
Table of Contents
1. Physical Properties of Benzene
The Kekulé structure of benzene, a six-carbon ring with alternating single and double bonds, cannot explain its properties. Benzene is more stable than expected, its NMR spectrum is unusual, and its bond lengths are intermediate between single and double bonds. It is also more reactive than simple hydrocarbons.
These properties suggest that benzene has a hybrid structure, with delocalized electrons.
Table 1 give important physical properties for benzene:
| Property | Value |
|---|---|
| Molecular weight | 78.11 g/mol |
| Odor | Sweet |
| Color | Colorless |
| Density at 20 °C | 0.879 g/cm³ |
| Melting point | 5.53 °C |
| Boiling point | 80.01 °C |
| Refractive index at 20 °C | 1.501 |
| Critical density | 0.309 g/cm³ |
| Critical pressure | 48.9 bar |
| Critical temperature | 288.9 °C |
| Flash point | -11 °C |
| Ignition temperature | 595 °C |
| Explosion limits in air (vol%) | 1.4–6.7 |
| Viscosity at 20 °C | 0.654 mPa·s |
| Solubility in water | Slightly soluble |
| Solubility in organic solvents | Miscible with most organic solvents |
2. Chemical Properties of Benzene
Benzene as the basic unit of aromatic compounds is a major industrial chemical with a wide range of applications. It is thermally stable and undergoes a variety of reactions, including substitution, addition, and dehydrogenation, to produce a myriad of useful products.
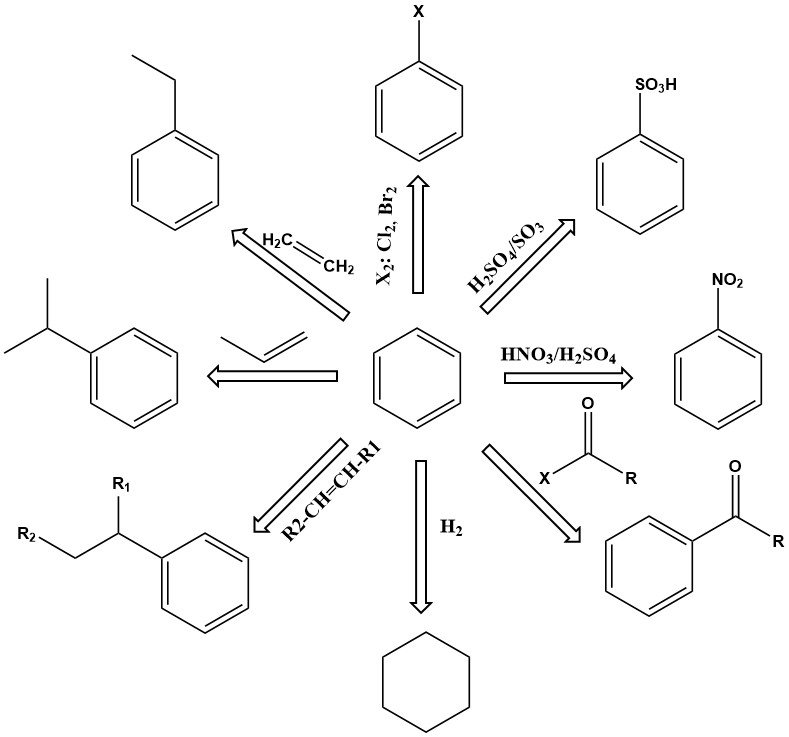
Substitution reactions of benzene are of primary importance. Depending on the reaction conditions, one or more of the hydrogen atoms in the benzene ring can be exchanged for nitro, sulfonic acid, amine, hydroxyl, chlorine, bromine, or other groups. Products include phenol, nitrobenzene, chlorobenzene, benzenesulfonic acid, and many others.
Other important reactions of benzene involve addition, such as alkylation and hydrogenation. These reactions generally take place at elevated temperature and pressure, sometimes requiring active catalysts.
For example, ethylbenzene is produced by the alkylation of benzene with ethylene in the presence of an aluminum chloride catalyst.
Cumene is another important primary addition product of benzene, produced by the vapor-phase catalytic alkylation of benzene with propene over phosphoric acid or kieselguhr catalysts at 200–250 °C and 2.7–4.2 MPa gauges pressure.
Hydrogenation is also an addition reaction. One of the most important is the hydrogenation of benzene to produce cyclohexane.
The dehydrogenation of ethylbenzene to styrene is an important commercial reaction. This endothermic reaction requires high temperature to proceed kinetically and thermodynamically.
Benzene is an essential feedstock for the production of many other important chemicals, including plastics, resins, detergents, and pharmaceuticals. Most of the reactions discussed in this chapter are primary reactions of benzene. A countless of reactions and products rely on benzene, either directly or indirectly, as their source.
3. Raw Materials for Benzene
Benzene and its homologues, such as toluene and xylenes, are found in crude oil in small amounts, making physical separation and recovery impractical. Crude oil fractions from Saudi Arabia and the United Kingdom have low benzene, toluene, and xylene (BTX) contents, but higher naphthene contents.
Heavier crudes and synthetic crudes from tar sands may have higher aromatic contents in the light naphtha fraction. An analysis of the C5-150 °C naphtha fraction from a conventional crude and a synthetic naphtha derived from Athabasca tar sands shows that the synthetic naphtha has lower aromatic and naphthene contents than the conventional crude fraction.
However, the higher naphtha fraction from the synthetic crude has a considerably higher aromatic content (32 versus 19 %), although the naphthene content is lower. This is likely due to dealkylation or ring opening of the higher molecular mass aromatics in the bitumen.
- Visit the link for a detailed article about the Production of Benzene →
4. Uses of Benzene
Benzene is used to produce ethylbenzene, cumene, and cyclohexane, which account for 75–80% of its consumption as a chemical feedstock.
Ethylbenzene is dehydrogenated to styrene, the monomer for important polymers such as acrylic-butadiene-styrene resins and styrene-butadiene rubber.
Cumene is used to produce phenol and acetone. Phenol is used mainly for phenolic resins.
Cyclohexane is used to produce adipic acid, which is used to make nylons.
About 5% of benzene is nitrated to nitrobenzene, which is then hydrogenated to aniline.
Benzene can also be oxidized to maleic anhydride, a precursor for polyester resins.
Other benzene products include halogenated benzenes and linear alkylbenzenes, which are used to make detergents.
5. Toxicology and Occupational Health of Benzene
Benzene is a toxic compound that can cause acute and chronic effects. It is absorbed through inhalation, ingestion, or skin absorption. Acute poisoning can lead to headaches, confusion, loss of muscular control, and irritation of the respiratory and gastrointestinal tract. Higher concentrations can lead to unconsciousness or death. Chronic exposure can lead to anemia and leukemia.
Inhalation is the primary source of benzene poisoning. Ingestion can cause irritation of the mouth, esophagus, and stomach. Skin absorption is not a significant source of poisoning.
Several studies have investigated the toxicity of benzene. It is selectively absorbed by the lungs and retained in various bodily tissues. Benzene is metabolized slowly, forming toxic intermediates. Individual susceptibility to poisoning varies.
Benzene exposure has been linked to leukemia, pancytopenia, and aplastic anemia. Further research is needed to determine the exact causal relationship.
Emissions of benzene come from motor gasoline, coal carbonization, and historically from benzene-containing solvents.
Prevention of benzene poisoning involves good laboratory and plant practices, minimizing concentrations in work areas, and using protective measures.
Regulatory bodies have established exposure limits for benzene and classified it as a potentially carcinogenic substance.
Recent regulations aim to reduce volatile organic compound emissions, including benzene, from petroleum refineries and chemical plants.
Ongoing studies and regulatory efforts reflect the active engagement in understanding and controlling the toxicity of benzene.
Continued research and stringent measures are needed to mitigate the risks associated with benzene exposure.
Reference
- Benzene; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a03_475
