Mandelic Acid: Properties, Production and Uses
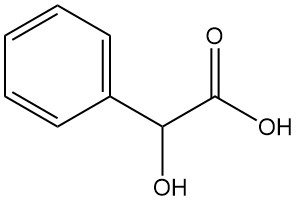
What is Mandelic Acid?
Mandelic acid, also known as α-hydroxyphenylacetic acid or phenylglycolic acid, is an aromatic alpha-hydroxy acid with the chemical formula C8H8O3. It is a white crystalline powder with a faint sweet odor.
Mandelic acid was first discovered in 1831 by the German pharmacist Ferdinand Ludwig Winckler. He isolated it by heating amygdalin, a compound found in bitter almonds, with diluted hydrochloric acid.
The name “mandelic acid” is derived from the German word “Mandel,” which means almond.
Table of Contents
1. Physical Properties of Mandelic Acid
Racemic (R,S)-mandelic acid is a colorless, crystalline solid that is readily soluble in ethanol and diethyl ether, less soluble in chloroform, and insoluble in petroleum ether. Alkaline solutions dissolve mandelic acid into corresponding salts.
Racemic (R,S)-mandelic acid can be separated into its enantiomers by reaction with cinchonine in an aqueous solution, which results in the precipitation of (S)-(+)-mandelic acid as a salt while (R)-(-)-mandelic acid remains in solution.
Alternatively, using S-(+)-aminodiol for the resolution process leads to the crystallization of (R)-(-)-mandelic acid, leaving the (S)-(+)-enantiomer in solution.
Table 1 summarizes the physical properties of mandelic acid.
| Property | (R,S)-Mandelic acid | (R)-(-)-Mandelic acid |
|---|---|---|
| CAS number | [611-72-3] | [611-71-2] |
| Chemical formula | C8H8O3 | C8H8O3 |
| Molecular weight | 152.15 g/mol | 152.15 g/mol |
| Melting point | 118-121 °C | 132-135 °C |
| Boiling point | 321.8 °C | |
| Density | 1.30 g/cm3 | |
| Refractive index | 1.5204 | |
| Solubility in water | 15 g/100 mL | |
| pKa | 3.41 | |
| Flash point | 163 °C | |
| Specific rotation (c = 5) | [α]D20 =-154 to -157° |
2. Chemical Reactions of Mandelic Acid
Mandelic acid turns brown when exposed to light. It reacts with bases to form salts, such as sodium mandelate.
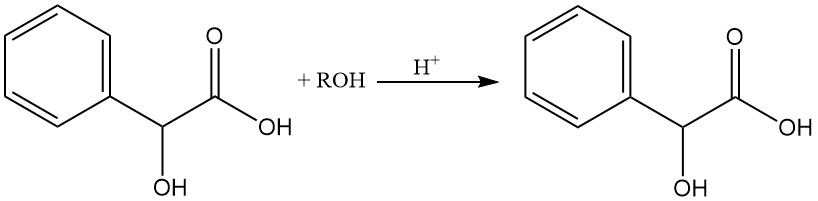
Mandelic acid can be esterified easily with alcohols in the presence of hydrogen chloride.
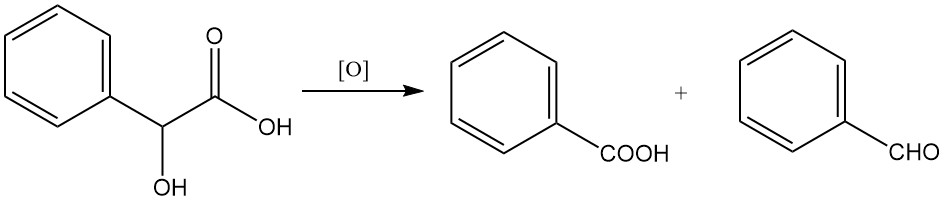
Oxidation with strong oxidizing agents like potassium permanganate or chromic acid can lead to the formation of benzaldehyde or benzoic acid.
Under specific conditions, mandelic acid can be decarboxylated to form benzene.
Mandelic acid can undergo typical aromatic substitution reactions such as halogenation, alkylation, acylation, nitration, and sulfonation.
3. Production of Mandelic Acid
Racemic (R,S)-mandelic acid is produced by the hydrolysis of mandelonitrile using hydrochloric acid.
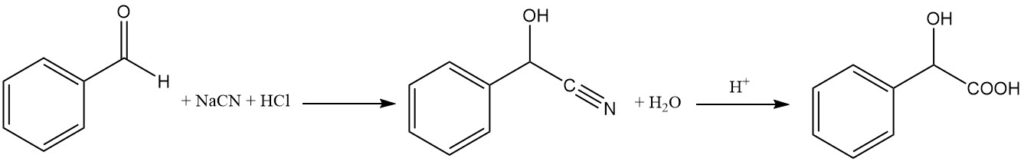
Mandelonitrile is prepared by the reaction of benzaldehyde and nascent hydrogen cyanide (NaCN + HCl) at a temperature below 10 °C. Sodium bisulfite is used as a catalyst. The resulting mandelonitrile is an oily liquid that is separated from the aqueous phase.
The obtained mandelonitrile is treated with concentrated hydrochloric acid at room temperature for about 12 hours, and then the excess water and hydrochloric acid are evaporated. This step results in the formation of crude mandelic acid.
Crude mandelic acid is purified by either benzene or ether extraction. The solid residue containing mandelic acid is extracted multiple times with hot benzene or ether, and it is crystallized by cooling.
The typical yield of pure mandelic acid is around 50–52% based on benzaldehyde.
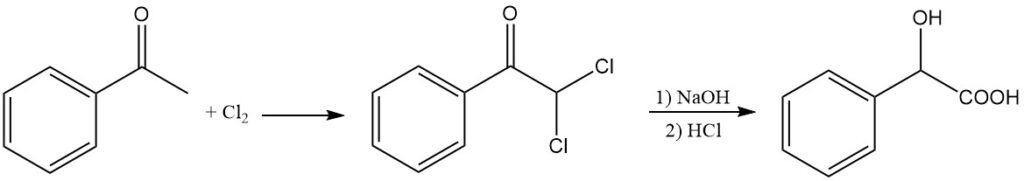
Mandelic acid can be synthesized alternatively by the alkaline hydrolysis of α,α-dichloroacetophenone. Acetophenone is reacted with chlorine to produce dichloroacetophenone, which undergoes alkaline hydrolysis by sodium hydroxide at 65 °C, followed by acidolysis with HCl.
(R)-Mandelic acid can be produced by a biosynthesis process that uses styrene, L-phenylalanine, glycerol, or glucose as starting materials. By combining multiple enzymatic steps, (R)-mandelic acid can be produced efficiently from these renewable feedstocks.
Additionally, integrating L-phenylalanine biosynthesis into the process enables the direct conversion of glucose or glycerol into (R)-mandelic acid within a single organism. This biocatalytic method offers a promising alternative to traditional chemical synthesis methods but is still in development.
4. Uses of Mandelic Acid
Mandelic acid is mostly used to produce its esters, which are analgesic, antirheumatic, and spasmolytic agents. For example, cis-3,5,5-trimethylcyclohexyl mandelate is used as a spasmolytic agent and vasodilator. Mandelic acid tropine ester (homatropine-HBr) is used in ophthalmology for pupil dilation.
Hexamethylenetetramine mandelate, also known as mandelamine, mandropine, diuramine, or hexydaline, is employed in the treatment of urinary tract infections.
Mandelic acid is also used in skincare for acne treatment, exfoliation, hyperpigmentation, anti-aging, and skin texture improvement.
Mandelic acid has bacteriostatic properties and is employed in the treatment of urinary tract infections. Administered orally as ammonium or calcium salts, it effectively inhibits the growth of Gram-negative bacteria and certain Gram-positive microorganisms.
Optimal antimicrobial activity is observed under acidic urine conditions (pH below 5.5). A 1% mandelic acid solution is used as a flushing solution for the maintenance of indwelling urinary catheters.
5. Toxicology of Mandelic Acid
Acute oral toxicity studies of mandelic acid in rats show an LDLo (oral, rat) of 3000 mg/kg.
Inhalation of mandelic acid may cause respiratory irritation, potentially leading to asthma-like symptoms, bronchitis, or pneumoconiosis upon prolonged exposure. Individuals with pre-existing respiratory conditions are at increased risk.
Mandelic acid is a severe eye irritant.
Dermal contact may induce skin inflammation or exacerbate existing dermatitis. Avoid contact with open wounds.
Repeated or prolonged exposure may result in kidney damage and cumulative health effects.
References
- Hydroxycarboxylic Acids, Aromatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a13_519
- Method for synthesizing mandelic acid. – https://patents.google.com/patent/CN112321410A/en
- https://bioresourcesbioprocessing.springeropen.com/articles/10.1186/s40643-021-00374-6
- https://www.orgsyn.org/demo.aspx?prep=CV1P0336
- https://pubs.rsc.org/en/content/articlelanding/2017/ra/c6ra25562k
- https://www.sciencedirect.com/science/article/abs/pii/S1075628002290072
- https://patents.google.com/patent/DE2936416A1/en
- https://www.lgcstandards.com/DE/en/Resources/Articles/Pharma_roots_mandelic_acid
- https://datasheets.scbt.com/sc-205481.pdf
