Malonic Acid: Properties, Reactions, Production and Uses
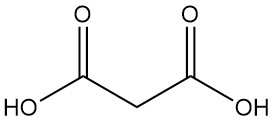
What is Malonic Acid?
Malonic acid, also known as propanedioic acid or methanedicarboxylic acid, is a dicarboxylic acid with the chemical formula C3H4O4. It is a colorless, hygroscopic solid that sublimes in a vacuum. The ionized form of malonic acid, as well as its esters and salts, are known as malonates.Table of Contents
1. Physical Properties of Malonic Acid
Malonic acid is a white, hygroscopic solid that is very soluble in water (139 g in 100 g of water at 22 °C) and soluble in pyridine (15 g in 100 g of pyridine at 15 °C). It is slightly soluble in ethanol and diethyl ether and insoluble in benzene.
The physical properties of malonic acid are listed in Table 1.
| Property | Value |
|---|---|
| CAS registry no. | [141-82-2] |
| Molecular formula | C3H4O4 |
| Molecular weight | 104.06 g/mol |
| Melting Point | 134 – 138 °C |
| Boiling point | Decomposes at 140 °C |
| Density | 1.62 g/cm³ |
| pKa1 | 2.83 |
| pKa2 | 5.70 |
| Flash point | 157 °C |
2. Reactions of Malonic Acid
Malonic acid is found in small amounts in sugar beet and green wheat and is formed by the oxidative degradation of malic acid.
Malonic acid exhibits reactions typical of carboxylic acids and high reactivity of the central methylene group because of the acidity of the hydrogen atoms at the 2-position.
Malonic acid reacts like most carboxylic acids to produce amides, esters, anhydrides, and acid chlorides.
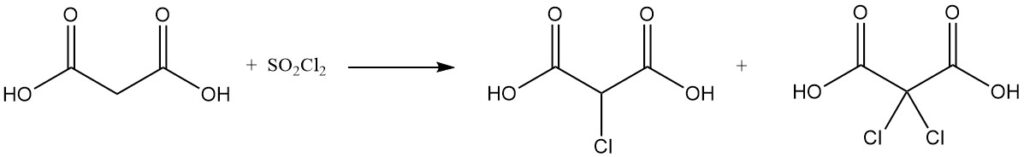
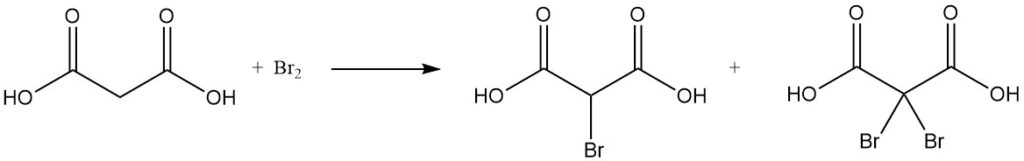
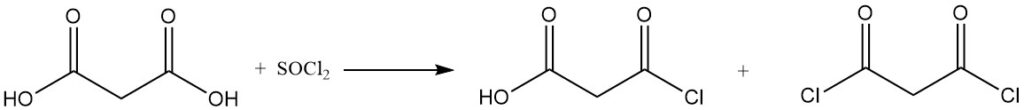
The reaction of malonic acid with sulfuryl chloride or bromine yields mono- or dihalogenated derivatives, while thionyl chloride or phosphorus pentachloride forms mono- or diacyl chlorides.
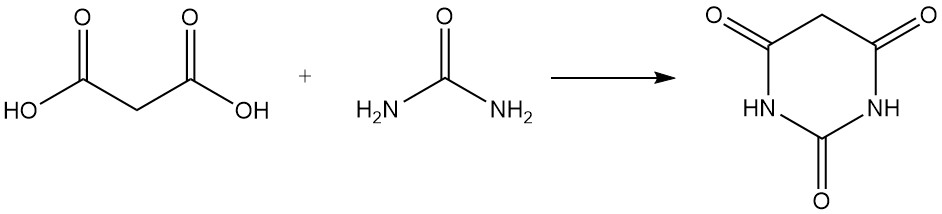
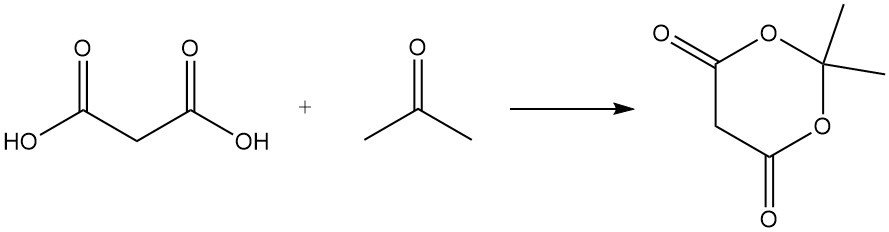
Malonic acid reacts with urea to produce barbituric acid, a precursor to various drugs, and with acetone to form Meldrum’s acid, an important intermediate in organic synthesis.
Unlike typical carboxylic acids, heating malonic acid with phosphorus pentoxide doesn’t produce an anhydride but rather carbon suboxide, a toxic gas that readily reforms malonic acid upon contact with water.
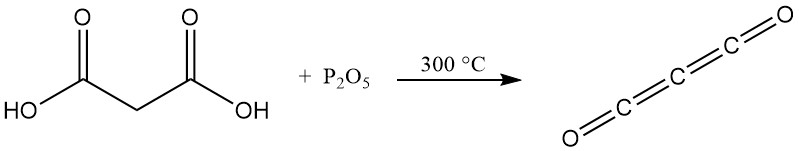
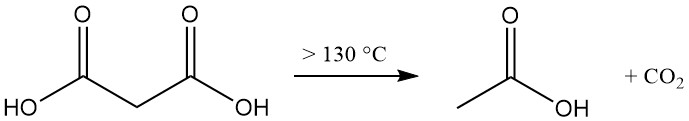
Thermal decomposition of malonic acid above 130 °C (free acid) or 70 °C (aqueous solution) produces acetic acid and carbon dioxide.
The mono- and dianions of malonic acid are more stable compared to the free acid. In aqueous solutions, monosodium malonate decomposes above 90 °C, while disodium malonate decomposes above 130 °C.
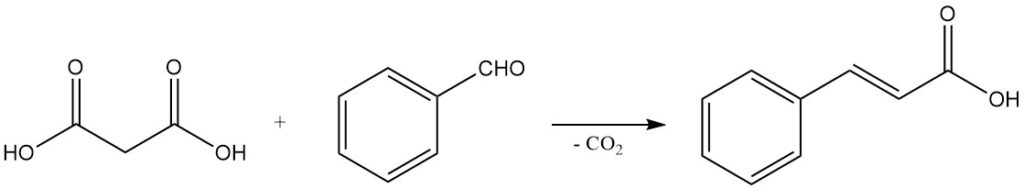
The mild decarboxylation behavior, coupled with the ability to undergo Knoevenagel condensations, makes malonic acid an important reagent for synthesizing α,β-unsaturated carboxylic acids. For example, reaction with benzaldehyde yield cinnamic acid, and reactions with aliphatic aldehydes produce acrylic acids.
Research has also explored platinum complexes of malonic acid and its derivatives as potential antitumor agents.
3. Industrial Production of Malonic Acid
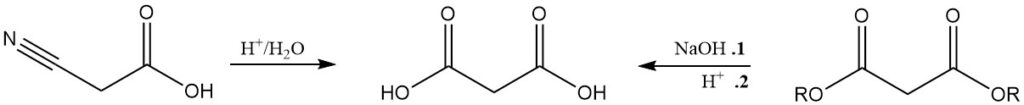
Although less significant than malonate production, malonic acid is commonly produced by the hydrolysis of cyanoacetic acid or by the acid saponification of malonates.
Emerging methods have also been reported for malonic acid production, which include ozonolysis of cyclopentadiene, palladium-catalyzed air oxidation of 1,3-propanediol, metal-catalyzed oxidation of 3-hydroxypropionaldehyde or 3-hydroxypropionic acid and biocatalytic conversion of malononitrile using a nitrilase enzyme.
The first laboratory synthesis of malonic acid, achieved in 1858, involved the oxidative decomposition of malic acid using potassium dichromate. This method is not commonly used today.
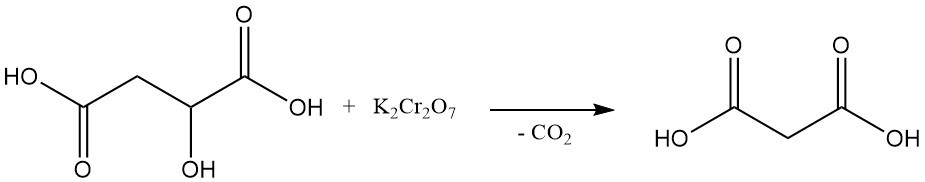
4. Uses of Malonic Acid
Malonic acid is used as a building block for various organic compounds due to its ability to introduce an acetic acid moiety under mild conditions via Knoevenagel condensation followed by decarboxylation. Examples of compounds synthesized from malonic acid include:
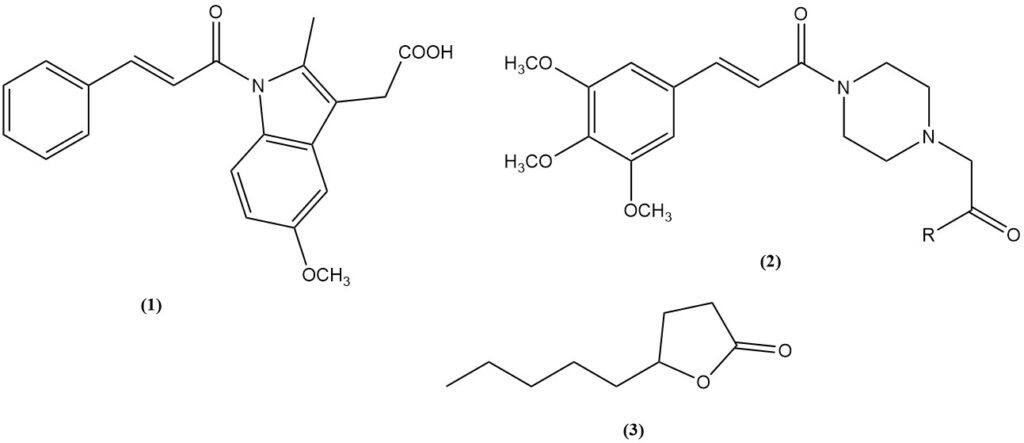
- Cinnamic acid is a precursor to the anti-inflammatory drug cinmetacin (1).
- 3,4,5-trimethoxycinnamic acid: An intermediate for the vasodilators cinepazet (2, R = OEt) and cinepazide (2, R = 1-pyrrolidinyl).
- γ-Nonanoic lactone (3): A fragrant compound obtained through Knoevenagel condensation of malonic acid with heptaldehyde followed by cyclization.
Malonic acid is also used in the production of engineering plastics with desired properties, biodegradable containers, barbiturates, and coatings.
5. Toxicology of Malonic Acid
Malonic acid is considered to have moderate toxicity, primarily affecting the eyes and the skin. Here’s what toxicology data is available:
Acute Oral Toxicity: LD50 (Oral, rat): 2750 mg/kg
Malonic acid can irritate the skin (mild skin irritation in rabbits) and cause serious eye damage upon contact. Safety Data Sheets (SDS) recommend wearing appropriate personal protective equipment (PPE) to avoid contact.
There is no readily available data on chronic health effects in humans.
Inhalation of malonic acid dust may also be harmful, although specific data may be limited.
The specific effects of malonic acid exposure can vary depending on factors like the amount, duration of exposure, and route of entry (inhalation, ingestion, skin contact).
As with any chemical, it’s crucial to handle malonic acid according to recommended safety protocols outlined in Safety Data Sheets (SDS).
References
- Malonic Acid and Derivatives; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a16_063.pub2
- Malonic acid. – https://www.acs.org/molecule-of-the-week/archive/m/malonic-acid.html
- The Knoevenagel Reaction. – https://www.sciencedirect.com/science/article/abs/pii/B9780080523491000330
- https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-m/S25416.pdf
