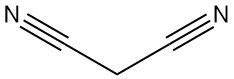
What is Malononitrile?
Malononitrile, also known as propanedinitrile, malonic acid dinitrile, or dicyanomethane, is an organic compound with the chemical formula C3H2N2. It is a colorless or white solid that is a valuable building block used extensively in organic synthesis.
Table of Contents
1. Physical Properties of Malononitrile
Malononitrile forms colorless crystals that are soluble in polar solvents like water, lower alcohols, acetonitrile, and diethyl ether, but it is insoluble in non-polar solvents such as tetrachloromethane, petroleum ether, and xylene.
Prolonged storage can induce color change, transforming the crystals from colorless to yellow or even dark brown without compromising their quality.
Malononitrile is toxic if ingested, and can cause severe skin and eye irritation.
The physical properties of malononitrile are listed in Table 1.
| Property | Value |
|---|---|
| CAS registry no. | [109-77-3] |
| Molecular formula | C3H2N2 |
| Molecular mass | 66.06 g/mol |
| Melting point | 31.6 °C |
| Boiling point | 218 – 219 °C |
| Density (20 °C) | 1.19 g/mL |
| Refractive index (34 °C) | 1.4146 |
| Dipole moment (25 °C) | 3.57 D |
| pKa | 11.2 |
| Vapor Density | 2.3 |
| Vapor Pressure | 0.2 mmHg |
| Flash point | > 86 °C |
| Solubility (20 °C) | |
| water | 133 g/L |
| diethyl ether | 200 g/L |
| ethanol | 400 g/L |
2. Chemical Reactions of Malononitrile
Malononitrile exhibits versatile reactivity due to the nucleophilicity of the malononitrile anion, which can be obtained by deprotonation with a weak base, and the electrophilic nature of its cyano groups.
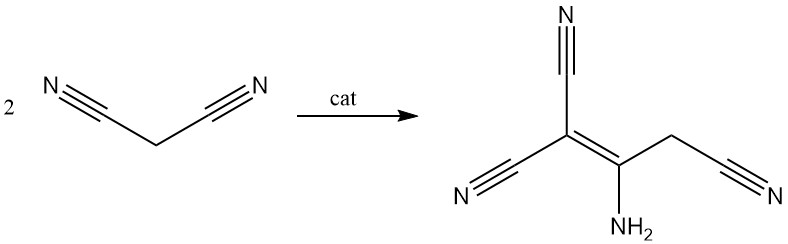
Malononitrile is dimerized in the presence of a base to yield 2-amino-1,1,3-tricyano-2-propene.
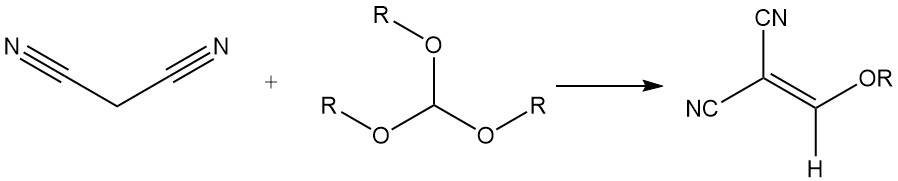
It reacts with trialkyl orthoformates to produce alkoxymethylene malonitriles, which are used for the synthesis of styryl dyes.
Dialkylation of malononitrile with alkyl halides and acylation with acid chlorides/anhydrides is possible.
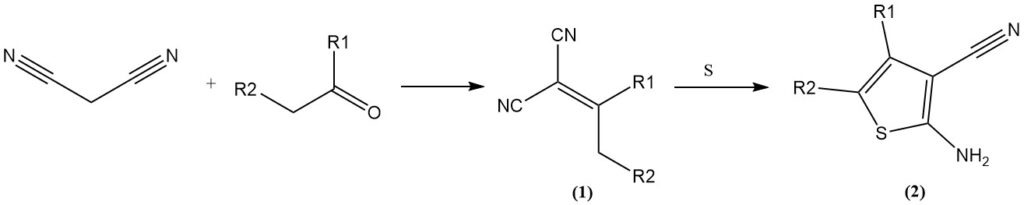
Knoevenagel condensation with aldehydes/ketones yields ylidene malonitriles (1), which can undergo further reactions. Examples include the reaction with elemental sulfur (Gewald reaction) to form 3-cyano-2-amino-thiophenes (2) or the cycloaddition with azides to yield tetrazoles.
Malononitrile reacts with bromine to yield mono- or dibrominated derivatives. Thermal decomposition of the dibromide complex affords tetracyanoethylene.
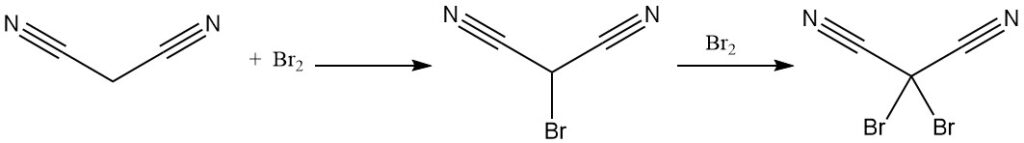
Reaction of dibromomalononitrile with potassium cyanide yields the potassium salt of tricyanomethane, also produced from malononitrile and cyanogen chloride. Tricyanomethane is used in ionic liquid synthesis.
The reaction of malononitrile with carbon disulfide in the presence of a base produces salts of (dimercaptomethylene)malonitrile, a precursor for antimicrobials. Dimethylation yields another important building block for heterocycles.
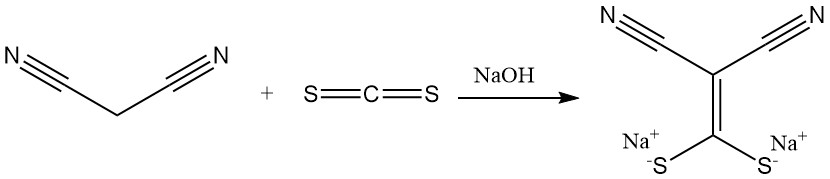
Cyanothioacetamide is prepared by reaction with hydrogen sulfide.
Acid-catalyzed reactions of malononitrile with alcohols generate imidates (3), which are starting materials for nitrogen heterocycles like 4,6-dimethoxypyrimidines (4) or bis(oxazolines) (5).
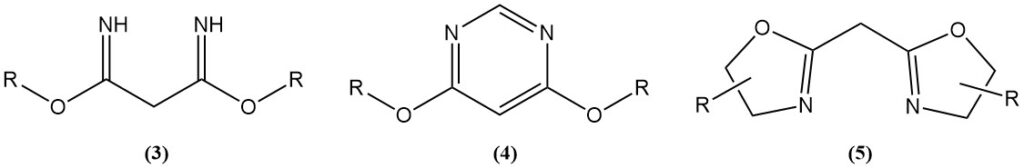
Bis (oxazolines) are produced by Ritter-type reactions or by using a catalyst. Bis(oxazolines) synthesized from malononitrile or its derivatives and chiral aminoalcohols are used as ligands in asymmetric transition metal-catalyzed reactions.
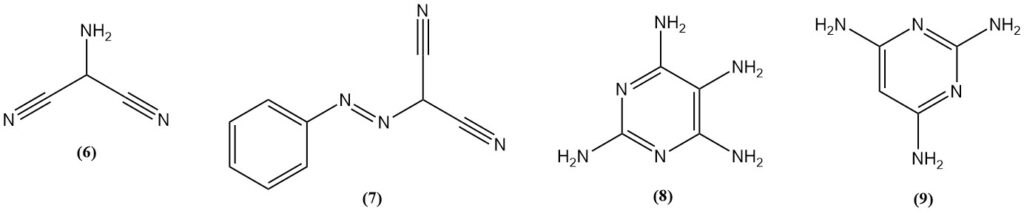
2-Aminomalononitrile (6) is obtained by nitrosation of malononitrile, followed by hydrogenation or by reduction of (phenylazo)malononitrile (7). Aminomalononitrile reacts with guanidine to yield 2,4,5,6-tetraaminopyrimidine (8).
2,4,6-triaminopyrimidine (9) can be prepared by the reaction of malononitrile and guanidine, while 2,4,6-triaminopyrimidine is produced from formamidine.
3. Production of Malononitrile
Until the 1970s, malononitrile was produced via a batch process by the dehydration of cyanoacetamide using phosphorus pentachloride or phosphorus oxychloride. This method, while documented by patents even recently, is no longer commercially used except in some small-scale Chinese plants.
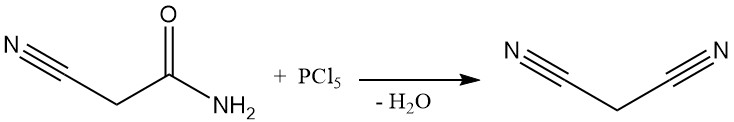
The dominant industrial method for malononitrile synthesis today is a continuous, high-temperature reaction between acetonitrile and cyanogen chloride. This process uses a tube reactor operating above 700 °C and achieves nearly complete conversion in a single pass, yielding malononitrile.
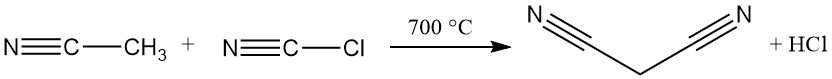
Excess acetonitrile, hydrochloric acid, and trace amounts of maleic, succinic, and fumaric acids are present with the desired product as impurities.
The product exiting the reactor is rapidly cooled to quench the reaction and separate gaseous hydrogen chloride, which is then recovered through scrubbing. Excess acetonitrile is removed via a combination of vacuum distillation and thin-film evaporation, allowing for its recycling with minimal contamination.
Separation of maleic and fumaric acids from the crude malononitrile is impractical due to their similar boiling points. Therefore, these impurities are converted into higher-boiling compounds through a Diels-Alder reaction in a separate reactor. Finally, two vacuum distillations remove these byproducts, resulting in a 66% yield of malononitrile based on both starting materials.
The first industrial plant using this process was operated in 1973 by Lonza in Visp, Switzerland.
While alternative methods for malononitrile production have been proposed, such as those using cyanoacetates, malonates, or reactions with ammonia and various catalysts, none of these methods are currently employed on an industrial scale.
4. Uses of Malononitrile
Malononitrile is extensively used as a raw material in the production of vitamins, agrochemicals, pharmaceuticals, and dyes.
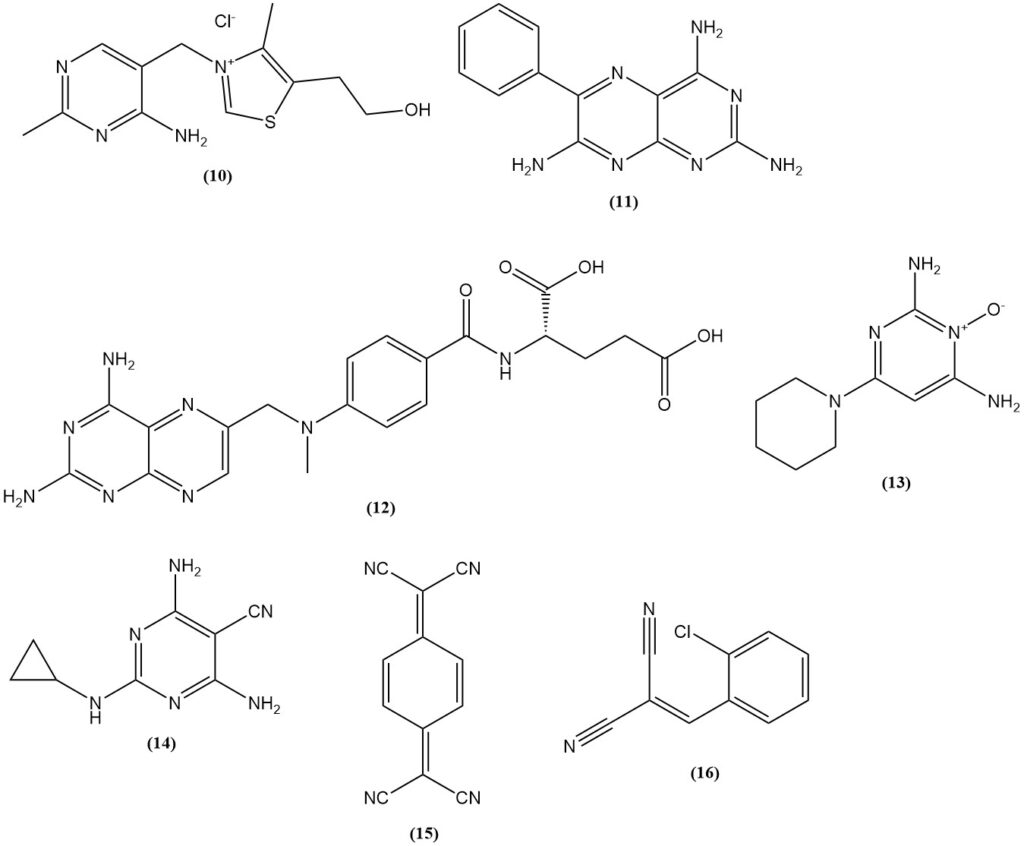
Malononitrile is used in the synthesis of thiamine (10) (vitamin B1), sulfonylurea herbicides based on 2-amino-4,6-dimethoxypyrimidine, such as benzsulfuron-methyl and halosulfuron-methyl and pharmaceuticals (the diuretic triamterene (11), the folic acid antagonist methotrexate (12), the antihypertensive minoxidil (13), and the pesticide dicyclanil (14)).
Examples of dyes and pigments produced from malononitrile include yellow methine dyes with a N,N-dialkylaminoaryl group (aminoarylneutrocyanines) like C.I. Disperse Yellow 90; blue aminoarylneutrocyanine dyes like C.I. Disperse Blue 354; and 1,4,5,8-naphthalenetetracarboxylic acid, which is a precursor for perinone pigments.
It is also used in the production of electrical conductors such as charge-transfer salts of 7,7,8,8-tetracyanoquinodimethane (15) (TCNQ) and the tear gas (o-chlorobenzylidene)malonitrile (16), which can be produced from the condensation of o-chlorobenzaldehyde with malononitrile.
5. Toxicology of Malononitrile
Malononitrile can cause severe eye irritation in rabbits upon exposure to 5 mg for 24 hours.
Malononitrile is highly toxic by various routes, with oral LD50 values ranging from 14 mg/kg to 69.8 mg/kg in rats. Common effects include convulsions, muscle weakness, respiratory depression, and behavioral changes.
Skin contact with malononitrile can also be lethal in rats at doses of 350 mg/kg. The available data suggests potential effects on various organs, including the lungs, blood, and nervous system.
Repeated oral exposure in rats at lower doses (285 mg/kg/19 weeks) can cause liver and weight changes.
Occupational exposure limits exist to regulate workplace air concentrations of malononitrile (e.g., NIOSH Recommended Exposure Level: 3 ppm).
References
- Malonic Acid and Derivatives; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a16_063.pub2
- https://pubchem.ncbi.nlm.nih.gov/compound/Malononitrile
- https://www.cdc.gov/niosh-rtecs/OO3010B0.html

