4-Methyl-2-pentanone: production, reactions and uses

4-Methyl-2-pentanone is a colorless liquid exhibiting a less discernible ketone-like aroma.
It has poor solubility in aqueous media but shows compatibility with conventional organic solvents. It can form an azeotrope with water as well as with a substantial number of solvents.
As an illustration, the azeotrope formed with water (boiling point of 87.9 °C) comprises 75.7 wt % of 4-methyl-2-pentanone, whereas that formed with n-butanol (boiling point of 114.4 °C) contains 70 wt % of 4-methyl-2-pentanone.
Table of Contents
1. Production of 4-Methyl-2-pentanone
The industrial synthesis of 4-Methyl-2-pentanone can be achieved via two processes using either acetone or isopropyl alcohol as a starting material:
1.1. A three-step process starting from acetone through diacetone alcohol and mesityl oxide, with the subsequent hydrogenation
In the initial stage, acetone undergoes condensation under the action of an alkali catalyst. Subsequently, the diacetone alcohol thus formed undergoes dehydration in the liquid phase over an acid catalyst, such as phosphoric or sulfuric acid, at a temperature range of 90 – 130 °C, yielding mesityl oxide with high selectivity.
Selective hydrogenation of the carbon – carbon double bond present in mesityl oxide can be carried out using various catalysts such as palladium in both the gas and liquid phases. This transformation exhibits exceptional selectivity.
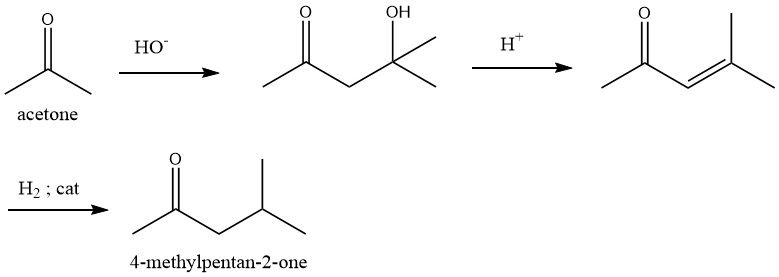
Dehydration and hydrogenation processes can be combined into a single step for the synthesis of 4-Methyl-2-pentanone.
1.2. A two-step process using acetone through mesityl oxide
This process is of less industrial significance, wherein acetone is converted to mesityl oxide in the first step and subsequently hydrogenated to 4-methyl-2-pentanone in the second step.
Copper chromite or zirconium phosphate are preferred catalysts for the condensation reaction, and palladium on aluminum oxide for the hydrogenation reaction.
Byproducts such as 4-methyl-2-pentanol can also be formed.
1.3. A one-step process from acetone and hydrogen
The single-step production of 4-methyl-2-pentanone uses a combination of cation exchanger with palladium as a catalyst.
The reaction is carried out at 130 °C and 0.5 – 5.0 MPa, wherein acetone and hydrogen are fed over a palladium-charged catalyst. This process displays a selectivity of over 95% at a conversion of less than 50%.
The production of 4-methyl-2-pentanone as a byproduct in the dehydrogenation of isopropyl alcohol, leading to increased production of acetone, has gained importance in recent times.
2. Chemical reactions of 4-Methyl-2-pentanone
4-Methyl-2-pentanone can undergo autoxidation to form peroxides. Studies have shown that upon evaporation of 4-methyl-2-pentanone-water mixtures under air, the aqueous phase exhibits a dangerous and increasing concentration of peroxide.
Peroxide can also be directly obtained by oxidation with 50% hydrogen peroxide in the presence of acids.
In condensation reactions with carbonyl compounds like acetone, the α-methyl group is usually reactive. However, only formaldehyde can undergo condensation reactions with the α-methylene group.
Industrial processes like hydrogenation and reductive amination may be carried out with the keto group.
3. Uses of 4-Methyl-2-pentanone
The primary application of 4-methyl-2-pentanone is as a solvent for vinyl, epoxy, and acrylic resins, natural resins, and nitrocellulose.
Moreover, it is a versatile extracting agent that finds use in the production of antibiotics or the elimination of paraffins from mineral oil to generate lubricating oils.
The significance of 4-methyl-2-pentanone as an intermediate for synthesis is relatively low. The most crucial product derived from this compound is 4-methyl-2-pentanol, which is obtained via hydrogenation of the ketone.
In addition, 4-methyl-2-pentanone peroxide has some importance as a polymerization initiator for ethylene and as a hardener for unsaturated polyester resins.
Reference
- Ketones; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a15_077
