Phenoxy Herbicides: Production, Uses and Toxicology
During World War II, research on chlorinated phenoxy compounds led to the discovery of 2,4-dichlorophenoxyacetic acid (2,4-D) as a selective herbicide. This discovery revolutionized weed control in grasses and related crops like cereals, rice, and sugarcane.
Patents issued before 1950, particularly by American Chemical Paint Co. (now Union Carbide) and Dow Chemical, documented this early development.
Despite patent expiration and competition from newer herbicides, chlorophenoxy compounds remain a significant global market segment. In 1982, the estimated manufacturing capacity for chlorophenoxyalkanoic acids and derivatives reached nearly 200,000 tons. However, weak prices and reduced demand led to a 10% decrease in worldwide production by 1983.
Table of Contents
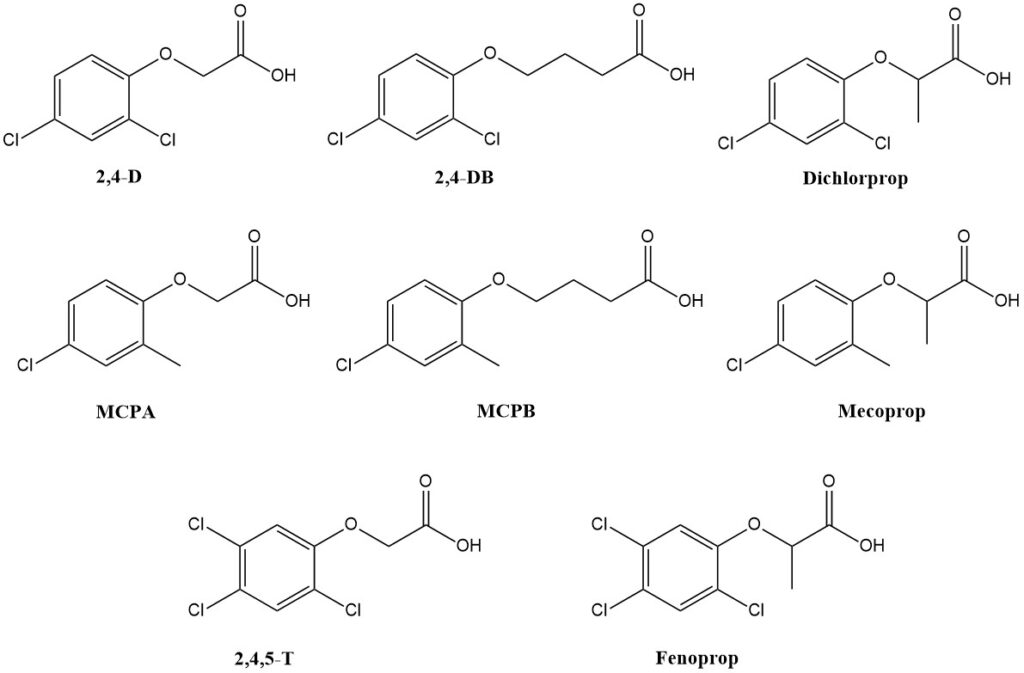
Table 1 lists the most prominent chlorophenoxyalkanoic acid herbicides. These are formulated as water-soluble salts or oil-soluble/emulsifiable esters.
2,4-D and MCPA are the major products by volume, followed by mecoprop and dichlorprop. Production of 2,4,5-T and fenoprop has largely ceased due to concerns about dioxin contamination arising during their manufacturing processes.
| Common Name | Chemical Name (IUPAC) | CAS Number | Formula | Molecular Weight (g/mol) |
|---|---|---|---|---|
| 2,4-D | (2,4-dichlorophenoxy)acetic acid | [94-75-7] | C8H6Cl2O3 | 221.04 |
| 2,4-DB | 4-(2,4-dichlorophenoxy)butyric acid | [94-82-6] | C10H10Cl2O3 | 249.09 |
| Dichlorprop | 2-(2,4-dichlorophenoxy)propionic acid | [120-36-5] | C9H8Cl2O3 | 235.05 |
| MCPA | (4-chloro-o-tolyloxy)acetic acid | [94-74-6] | C9H9ClO3 | 200.63 |
| MCPB | 4-(4-chloro-o-tolyloxy)butyric acid | [94-81-5] | C11H13ClO3 | 228.68 |
| Mecoprop | 2-(4-chloro-o-tolyloxy)propionic acid | [93-65-2] | C10H11ClO3 | 214.66 |
| 2,4,5-T | (2,4,5-trichlorophenoxy)acetic acid | [93-76-5] | C8H5Cl3O3 | 225.49 |
| Fenoprop | 2-(2,4,5-trichlorophenoxy)propionic acid | [93-72-1] | C9H7Cl3O3 | 269.53 |
1. Industrial Production of Phenoxy Herbicides
The Pokorny Process, first described in 1941, remains the dominant method for producing technical-grade chlorophenoxy acids. This process involves reacting a chlorophenol with a chloroalkanoic acid under alkaline conditions (around 100°C) in either an aqueous medium or an organic solvent like toluene, xylene, or chlorobenzene. The reaction can be summarized as follows:
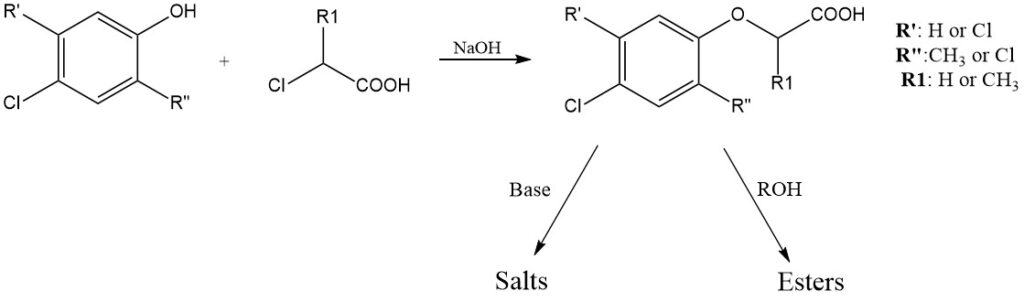
The resulting salt is then hydrolyzed to yield the free acid, which is further purified through recrystallization with an organic solvent or steam distillation. Chlorophenoxy acids derived from 2-chloropropionic acid contain an asymmetric carbon and are isolated as racemic mixtures.
A schematic representation of the Pokorny process for producing 2,4-D, its salts, and esters is illustrated in Figure 1.
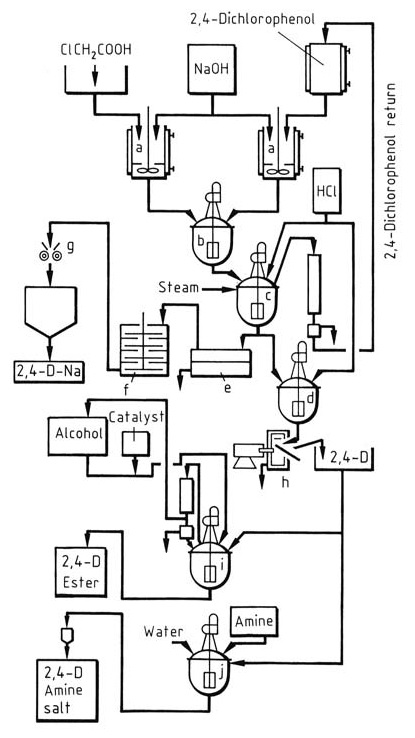
Figure 1: Production of 2,4-dichlorophenoxyacetic acid (2,4-D), as well as its salts and esters
a) Neutralization vessel; b) Reaction kettle; c) Acidification kettle; d) Precipitation vessel; e) Suction filter; f) Drier; g) Mill; h) Filter; i) Esterification kettle; j) Amination kettle
The type and quantity of byproducts formed during the Pokorny Process heavily depend on the purity of the starting chlorophenols. For example, 2,4-dichlorophenol, a precursor for 2,4-D, dichlorprop, and 2,4-DB, is typically obtained by phenol chlorination.
This process yields 2,6-dichlorophenol and 2,4,6-trichlorophenol as impurities. However, if chlorination is conducted in liquid sulfur dioxide below its boiling point, it can minimize these byproducts.
Similar considerations apply to MCPA, MCPB, and mecoprop production. These herbicides use chlorophenols derived from o-cresol, a readily available byproduct of coal tar distillation, particularly in Europe.
While chlorination primarily targets the 4th position of the molecule, substantial amounts of 6-chloro- and 4,6-dichloro-2-methylphenol are also formed when chlorine or alkali hypochlorites are used as chlorinating agents. Employing sulfuryl chloride for chlorination offers a solution for obtaining a pure product.
A different method is employed for the production of 2,4,5-trichlorophenol, which involves the hydrolysis of 1,2,4,5-tetrachlorobenzene using sodium hydroxide.
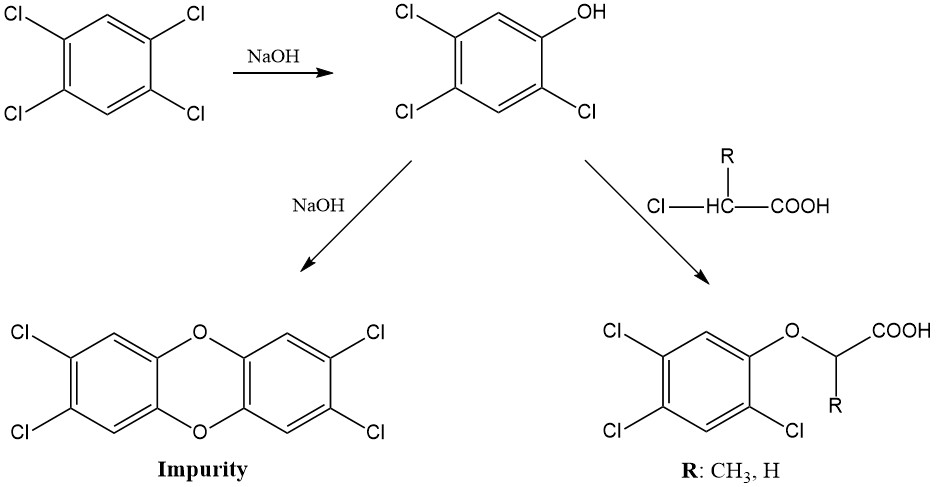
2,4-D can also be produced by chlorination of phenoxyacetic acid, but the byproduct 2,4-dichlorophenol is known for its strong odor. The chlorination of tolyloxyacetic acid yields primarily the desired 4-chloro derivative with minimal impurities.
For phenoxybutyric acid derivatives like 2,4-DB and MCPB, γ-butyrolactone is commonly used to acylate the chlorophenols. Another method is the formation of the appropriate chlorophenolate and reacting it with a chloroalkanoic acid ester.
The resulting phenoxy ester can be converted to free acid or used directly in oil-soluble herbicide formulations.
Water-soluble phenoxy acid salts are formed with various cations, including alkali metals, ammonia, and organic amines. Magnesium and calcium salts of 2,4-D and MCPA have lower water solubility compared to their sodium and potassium counterparts. Oil-soluble salts are derived from long-chain fatty acid amines.
Major producers of technical-grade chlorophenoxy acids and derivatives include Akzo Zout (Netherlands), BASF and Bayer (Germany), Chemie-Linz (Austria), Dow (US and Brazil), Koge (Denmark), A.H. Marks and May & Baker (UK), Rhône-Poulenc (France), and Vertac (US).
2. Uses of Phenoxy Herbicides

Phenoxy herbicides are primarily used for selective control of broadleaf weeds in cereal grains, pastures, and turf. They are also employed for brush removal in rangeland, forests, and non-cropland areas.
Application rates vary considerably, ranging from 0.25 kg/ha for grain crops (low dose) to up to 16 kg/ha for spot treatment of individual trees (high dose).
For specific plant growth regulation purposes in fruit orchards, very dilute solutions of 2,4-D and fenoprop derivatives can be used.
The choice of herbicide depends on the specific plants targeted for control. For instance:
- 2,4,5-T and fenoprop are effective against woody plants and herbaceous weeds resistant to 2,4-D.
- Dichlorprop, fenoprop, and mecoprop are used to control chickweed and other unwanted plants in lawns and turf.
- Butyric acid derivatives like 2,4-DB and MCPB are suitable for use on sensitive crops (peas, peanuts, soybeans, and legumes), as they become herbicidal only after undergoing β-oxidation to convert them to the acetic acid derivatives.
Phenoxy herbicides can be applied alone or in mixtures with other herbicides. Formulations include solutions, dispersions, or emulsions in water or oil. Application equipment generates large droplets to minimize spray drift.
While volatile derivatives like isopropyl and butyl esters of 2,4-D and 2,4,5-T are effective for weed and brush control in non-cropland areas, formulations with low-volatile long-chain esters and amine salts pose a lower risk of harming nearby crops or desirable vegetation.
For aquatic weed control, granular salt formulations of 2,4-D or fenoprop have been used, although longer-acting ester formulations offer better efficacy.
Distribution, marketing, and application of pesticides are subject to strict regulations in most countries. These regulations are enforced through national laws or by adhering to reviews conducted by expert committees under international organizations like the Food and Agriculture Organization (FAO), the World Health Organization (WHO), and the Codex Alimentarius Commission.
Labels on phenoxy herbicide containers undergo individual registration procedures in both exporting and importing countries, often even at the state level within a country. This detailed label specifies:
- The nature and amount of active ingredients.
- Necessary precautions to minimize risks to humans, livestock, wildlife, and the environment.
- Precise application conditions for each approved use.
Formulated phenoxy herbicide products often contain inert ingredients that prevent salt precipitation upon dilution with hard water or aid in dispersing or emulsifying esters in oil or oil-water mixtures. Oil concentrates, while less corrosive than emulsifiable concentrates or salt solutions, are more flammable and require stricter labeling for safe transport, storage, and handling.
3. Toxicology of Phenoxy Herbicides

Extensive studies have investigated the toxicology of chlorophenoxy herbicides in various animal models, including livestock, birds, fish, and laboratory animals. Additionally, pharmacokinetic studies in animals and humans have shown that these compounds are not bioaccumulative.
Chlorophenoxyalkanoic acids, their derivatives, and formulated products are classified as moderately toxic based on acute studies involving single high doses administered orally, dermally, or via inhalation in various animal species.
According to the World Health Organization (WHO) classification, 2,4-D and 2,4,5-T fall under class II (moderately hazardous), with acute oral LD50 values of 375 and 500 mg/kg body weight in rats, respectively.
Other phenoxy herbicides belong to class III (slightly hazardous), exhibiting acute oral LD50 values (mg/kg) in rats as follows: fenoprop (650), MCPB (680), 2,4-DB (700), MCPA (700), dichlorprop (800), and mecoprop (930). The acute oral toxicity of salts and esters is comparable to that of the parent acids on an acid-equivalent basis.
Dogs demonstrate higher sensitivity to chlorophenoxyalkanoic acids compared to rats, whereas birds are less susceptible. Livestock can tolerate even higher doses without apparent adverse effects. Phenoxy herbicides are not harmful to bees. The LC50 in fish can range from less than 1 mg/L for esters to exceeding 100 mg/L for acids and salts.
Dry forms of the acids cause only mild eye and skin irritation. However, prolonged or repeated contact with concentrated solutions or wetted powders may induce burns. Studies using rabbits exposed to dilute solutions of formulated products containing dimethylamine salt, isooctyl ester, or butyl ester of 2,4-D for three weeks did not reveal significant adverse systemic effects.
While phenoxy compounds do not exhibit acute toxicity in dermal and inhalation studies, occupational exposure is more likely to occur through these routes compared to ingestion.
It is crucial to take appropriate precautions to prevent skin contamination with technical-grade materials or concentrates and to avoid prolonged inhalation of vapor or dust. Occupational exposure limits for 2,4-D and 2,4,5-T have been set at 10 mg/m3 in both the United States (TWA) and Germany (MAK).
Signs and symptoms of acute overexposure in humans were observed only in cases of suicide by ingesting large quantities of 2,4-D products or due to significant dermal exposure resulting from poor occupational hygiene practices.
The acute no-observed-adverse-effect level (NOAEL) for biological effects in humans was estimated to be as high as 36 mg/kg body weight for 2,4-D or its equivalent in alkali salts, amine salts, or esters.
Studies involving repeated oral administration of 2,4-D, 2,4,5-T, MCPA, or fenoprop (as acids, salts, or esters) to livestock and laboratory animals (except dogs) at doses up to 50 mg/kg body weight showed minimal or no effects.
Reversible embryotoxic or fetotoxic effects were observed in the offspring of pregnant rats and mice given daily doses exceeding 40 mg/kg during organogenesis. However, true teratogenic effects, such as cleft palate, were only seen in mice administered 2,4,5-T, potentially attributable to high levels of TCDD contamination in certain technical-grade acid and ester samples.
Dietary feeding studies with phenoxy acids and derivatives in rats, dogs, and mice have been conducted for periods ranging from 3 months to 2.5 years to assess the potential for chronic toxicity, carcinogenicity, and reproductive effects. Key findings are:
- 2,4-D: Chronic feeding studies with 2,4-D showed no significant effects in rats up to a moderate dose (equivalent to 31 mg/kg/day). At a higher dose, offspring survival and weight were reduced.
- 2,4,5-T: Studies with purified 2,4,5-T caused effects in rats at high doses. However, studies with technical-grade material (containing impurities) did not.
- Other herbicides: Studies with fenoprop, MCPA, and mecoprop showed no significant effects at moderate doses. MCPA caused some deaths at the highest dose, but these were attributed to infections. Further studies are ongoing for MCPA.
The evidence for carcinogenicity is inconclusive for most chlorophenoxy herbicides except TCDD, which is classified in Group 2B (sufficient evidence for carcinogenicity in animals and inadequate evidence in humans and in short-term tests). There is limited evidence suggesting a potential carcinogenicity in humans from occupational exposure to chlorophenols and phenoxyacetic acid herbicides.
References
- Chlorophenoxyalkanoic Acids; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a07_009
- Phenoxy Herbicides (2,4-D); Hayes’ Handbook of Pesticide Toxicology. – https://www.sciencedirect.com/science/article/abs/pii/B9780123743671000847
