Gallic Acid: Properties, Reactions, Production and Uses
What is Gallic Acid?
Gallic acid, also known as 3,4,5-trihydroxybenzoic acid, is a hydroxyaromatic acid with the chemical formula C7H6O5. It is a white solid but often appears brown due to oxidation that is soluble in alcohol and polar solvents. It was discovered by Carl Wilhelm Scheele in 1786.
Gallic acid is found in the leaves of bearberry, in pomegranate root bark, gallnuts, witch hazel, sumac, tea leaves, oak bark, and many other plants, both in its free state and as part of the tannin molecule.
Table of Contents
1. Physical Properties of Gallic Acid
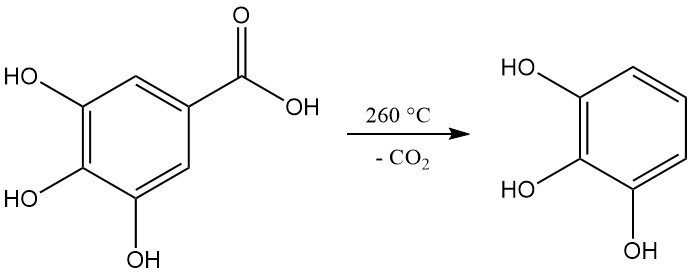
3,4,5-Trihydroxybenzoic acid, commonly referred to as gallic acid, appears as a white to pale yellow crystalline powder. Crystallization from aqueous solutions gives a monohydrate form in the shape of silky needles, which decomposes into pyrogallol and carbon dioxide upon heating to 258–263 °C.
Gallic acid is soluble in warm water, ethanol, diethyl ether, and acetone and insoluble in benzene and chloroform.
The physical properties of gallic acid are listed in the following table:
| Property | Value |
|---|---|
| CAS number | [149-91-7] |
| Chemical Formula | C7H6O5 |
| Molecular Mass | 170.12 g/mol |
| Melting Point | 258–263 °C (decomposition) |
| Sublimation Temperature | 210 °C |
| Density at 25 °C | 1.694 g/cm3 |
| pKa1 at 30 °C | 2.33 |
| pKa2 | 8.85 |
2. Chemical Reactions of Gallic Acid
The gallic acid molecule contains two functional groups: hydroxyl groups (phenolic) and a carboxylic acid group. With both functional groups having acidic hydrogens, they can react to form numerous esters, ethers, and salts, including digallic acid (1).

Gallic acid solutions, particularly those containing alkali metal salts, are susceptible to oxidation by atmospheric oxygen, resulting in a color change to brown like in pyrogallol.
Decarboxylation of gallic acid by heating to a temperature above 260 °C produces pyrogallol.
Gallic acid is a potent reducing agent that is capable of reducing gold or silver salts to their elemental forms.
The reaction of gallic acid with iron (III) salts produces an intense blue complex, which is used in ink dye formulation. Gallnut ink, composed of gallic acid and iron (II) sulfate, undergoes oxidation in the air to generate the blue iron (III)-gallic acid complex.
Heating gallic acid with concentrated sulfuric acid produces hexahydroxyanthraquinone (2; rufigallic acid) by condensation reaction.

The reaction with p-nitrosodimethylaniline hydrochloride forms oxazine derivatives.
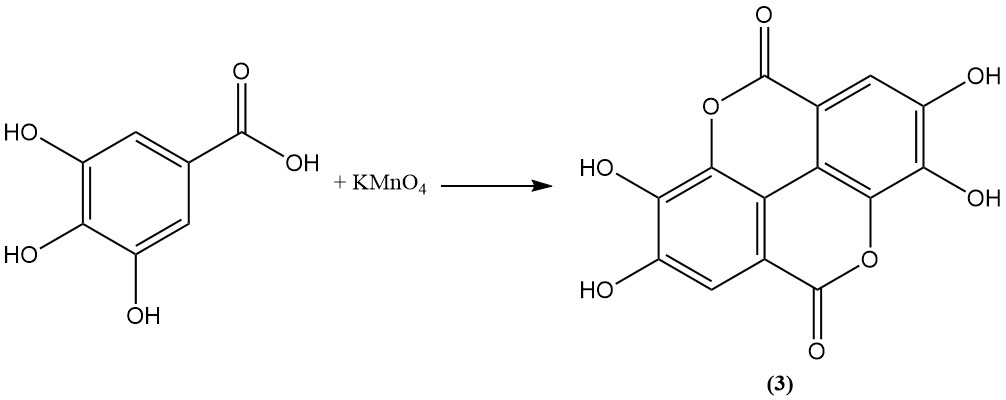
Oxidation of gallic acid using arsenic acid, permanganate, persulfate, or iodine leads to the formation of ellagic acid (3).
The carboxylic acid group of gallic acid can be esterified with alcohols by azeotropic esterification or by the Fischer method using alcohol with hydrochloric acid.

Ether-ester derivatives of gallic acid can be prepared by alkylation using dialkyl sulfates or alkyl halides in the presence of a base or by using diazomethane.
The hydroxyl group in the 4-position is more reactive compared to the others. Partial hydrolysis of 3,4,5-trimethoxybenzoic acid using strong acids produces 4-hydroxy-3,5-dimethoxybenzoic acid (4) (syringic acid).

3. Production of Gallic Acid
Gallic acid is a constituent of numerous tanning agents, and it exists in free form or is bound to tannins in natural sources such as divi-divi, oak bark, gallnuts, pomegranate roots, sumac, and tea.
Gallic acid is produced from tannin-rich aqueous gallnut extracts by acidic hydrolysis using sulfuric acid at 110–120 °C or by alkaline treatment.

It can also be prepared by enzymatic cleavage of tannin by tannase or by fermentation using molds like Penicillium glaucum and Aspergillus niger.
Solid-state or submerged fermentation processes using free or immobilized filamentous fungi, such as R. oryzae and Aspergillus foetidus, are developed to hydrolyze tannic acid from low-cost tara powder or other low-cost tannin-rich raw materials.
Gallic acid is then extracted from the fermentation solution by diethyl ether, which results in high yields of approximately 95%. This biosynthesis process consumes less energy compared to conventional acid hydrolysis.
4. Uses of Gallic Acid
Gallic acid is used in the production of iron gallnut ink and various dyes, including anthragallol, gallocyanine, galloflavin, and rufigallic acid.
Gallate esters, especially methyl gallate (gallicin) and propyl gallate, are used as antioxidants and food preservatives for fats.
Gallic acid is used as a reducing agent in pharmaceuticals (Dermatol, Airol and bismuth salt of gallic acid) and as a raw material for the production of the hallucinogenic alkaloid, mescaline, and trimethoprim (broad-spectrum antibiotic).
Gallic acid is used in the production of photographic developers, as is pyrogallol, which is used in oxygen absorption and azo dye synthesis.
It is also used in the leather tanning process due to its astringent properties and as an antioxidant and preservative in food products.
Many studies are investigating the potential health benefits of gallic acid as an antioxidant, antimicrobial, anti-inflammatory and anti-cancer agent.
5. Toxicology of Gallic Acid
Toxicity studies on gallic acid indicate low acute and subacute toxicity. However, the compound induced hemorrhagic effects in chicken embryos, emphasizing the need for dose limitations.
Reported LD50 values are 320 mg/kg (mouse, intravenous), 5000 mg/kg (rabbit, oral), and 4300 mg/kg (mouse, intraperitoneal). LDL0 values are 800 mg/kg (mouse, intraperitoneal) and 5 mg/kg (mouse, subcutaneous, 1 day pregnant)
Human exposure to gallic acid primarily occurs occupationally and through dietary sources.
It is classified as a mutagen and a teratogen.
References
- Hydroxycarboxylic Acids, Aromatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a13_519
- Organic and Fatty Acid Production, Microbial. – https://www.sciencedirect.com/science/article/abs/pii/B9780123739445001565
- Toxicity of natural products. – https://www.sciencedirect.com/science/article/abs/pii/B9780128243152011891
- https://pubchem.ncbi.nlm.nih.gov/compound/Gallic-Acid
