Aziridine: Properties, Reactions, Production and Uses

Aziridine, also known as ethylenimine, is a saturated heterocyclic amine with the formula C2H5N. It is a colorless, volatile liquid with a strong, unpleasant odor that is highly reactive due to the strain in its three-membered ring.
Commercially available aziridines and their derivatives are produced from ethylenimine or propylenimine.
Aziridine was first synthesized in 1888 by Gabriel, but was mistakenly named vinylamine at the time. It is synthesized by reacting 2-bromoethylamine hydrobromide with either silver oxide or potassium hydroxide.
Table of Contents
1. Physical Properties of Aziridine
Ethylenimine is a clear, colorless liquid with an amine-like odor. It is miscible with water and most organic liquids. When stored over solid sodium hydroxide, it is indefinitely stable.
Other properties of ethylenimine are shown in Table 1.
| Property | Value |
|---|---|
| Molecular weight (g/mol) | 43.07 |
| Density at 25 °C (g/mL) | 0.832 |
| Boiling point (bp) (°C) | 57 |
| Melting point (mp) (°C) | -74 |
| Vapor pressure at 25 °C (kPa) | 28.5 |
| Refractive index (25 °C) | 1.4123 |
| Viscosity at 25 °C (mPa·s) | 0.418 |
| Flash point (closed cup) (°C) | -11 |
2. Chemical Reactions of Aziridine
Aziridine is highly reactive compound that can undergo two main types of reactions: ring-preserving and ring-opening reactions.
Ring-preserving reactions involve processes such as alkylation or acylation of the nitrogen atom in the ring. Ring-opening reactions begin with the protonation of the nitrogen atom in the ring, followed by a nucleophilic attack on one of the carbon atoms.
Aziridine is often the most cost-effective and efficient way to incorporate an ethylamine group into a polymer or complex organic molecule.
2.1. Homopolymerization
Polyethylenimine (PEI) is synthesized by the homopolymerization of ethylenimine, catalyzed by acids, Lewis acids, or haloalkanes. The reaction is typically conducted at 90-110 °C in water or organic solvents. The resulting PEI has an average molecular mass of 10,000-20,000.
To obtain higher molecular mass PEIs, difunctional alkylating agents such as chloromethyloxirane or 1,2-dichloroethane can be added, or PEIs with a broad mass distribution can be ultrafiltered. Lower molecular mass PEIs can be obtained by incorporating a low molecular mass amine such as 1,2-ethanediamine during polymerization.
This yields a range of molecular masses from 300 to 106. PEI polymerization in organic solvents with cross-linking results in solid PEIs. PEI polymerization can also be performed on the surfaces of organic or inorganic materials, anchoring the PEI to the support.
All PEIs generated by these methods have highly branched structures with a roughly spherical shape. The distribution of amines in these polymers is approximately 30% primary, 40% secondary, and 30% tertiary, as determined by 13C NMR spectroscopy.
Specialized polymerization techniques can yield more linear or more branched PEI structures. Additionally, PEIs can be easily modified through polymer-analogous reactions to enhance their performance in specific applications.
2.2. Aziridine-Modified Polymers
Graft copolymers of aziridine can be synthesized by reacting ethylenimine with polymers that have carboxyl groups or amines in their structure. This improves the adhesion of the polymer to anionic surfaces or surfaces with hydroxyl groups.

Another method to introduce polyethylenimine-type side chains into other polymers is to (co-)polymerize monomers functionalized with oligoethylenimine side chains. This approach yields polymers with high charge densities without the need for carboxylic acids.
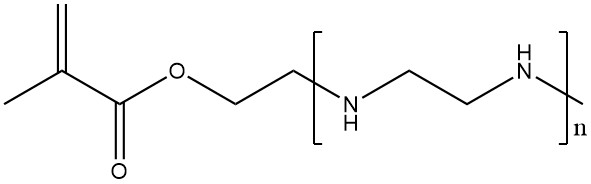
2.3. Polyfunctional Aziridines
Ethylenimine can react with tri-functional acrylates, such as 2-ethyl-2-(hydroxymethyl)-1,3-propanedioltriacrylate or 2,2-bis(hydroxymethyl)-1,3-propanediol triacrylate, to produce trifunctional aziridines. This reaction typically occurs without a solvent or catalyst, and the resulting products are clear, slightly viscous liquids.
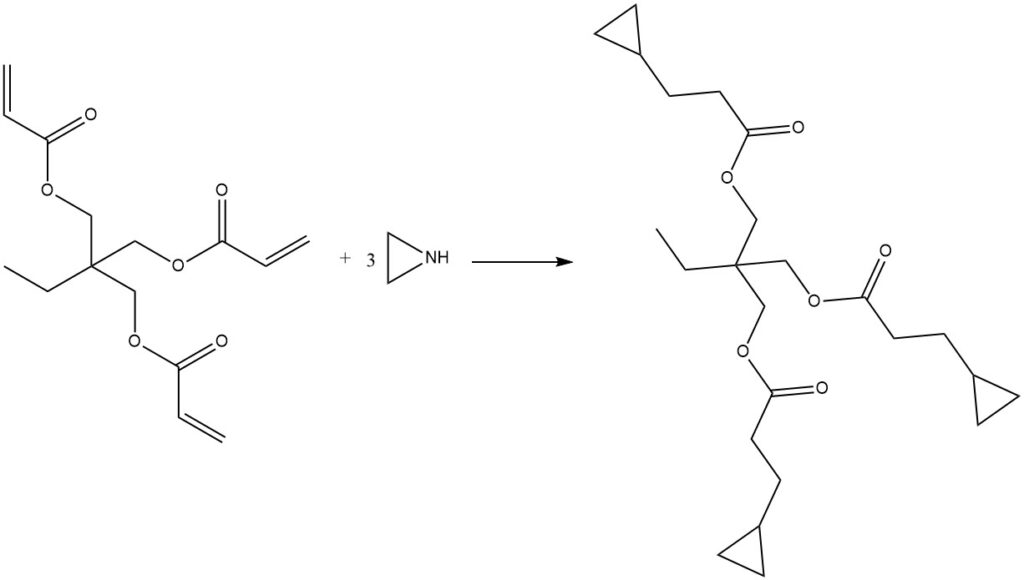
2.4. Other Reactions of Aziridine
Ethylenimine can react with hydrogen sulfide to produce thiols, and with thiols to produce aminoethyl sulfides. This reaction is used commercially to produce cysteamine, a raw material for the synthesis of pharmaceuticals, and 2,2′-thiobisethylamine.

Aziridine can also react with 2-mercaptoethanol to produce 2-[(2-aminoethyl)thio]ethanol, an intermediate for dye production, and with sulfurous acid to produce taurine, an essential additive for mammalian nutrition.
These are just a few examples of the industrially relevant ring-opening reactions of ethylenimine with sulfur nucleophiles.
Ethylenimine can also react with amines to produce asymmetrically substituted ethylenediamines. For example, N,N-dimethyl-1,2-ethanediamine can be produced by this reaction.

Aziridine can react with oxiranes, opening the oxirane ring while leaving the aziridine ring intact. This reaction can be used to produce 1-aziridine ethanol, for example.
Another commercial application of ethylenimine is the reaction with isocyanates to give iminoureas. For example, aziridine can react with 1,6-diisocyanatohexane to produce a polyurea.

3. Production of Aziridine
In the literature, four distinct industrial methods have been documented for the production of ethylenimine (aziridine):
- The beta-chloroethylamine process
- The Dow process
- The Wenker process by BASF
- The catalytic dehydration process of 2-aminoethanol by Nippon Shokubai
Now, only the latter two processes are in active use. As of 2006, the global annual production capacity for ethylenimine monomer is estimated to be approximately 9,000 metric tons.
Due to the monomer’s toxicity and high reactivity, the two primary producers, BASF and Nippon Shokubai, do not market the monomer directly but instead convert it into non-toxic polymers and intermediates.
3.1. The β-Chloroethylamine Process
From 1938 to 1963, ethylenimine was produced commercially in Germany by the reaction of 2-chloroethylamine hydrochloride with sodium hydroxide. This process, similar to the Dow Process, has the disadvantages of producing a corrosive chloride byproduct and a risk of contaminating the ethylenimine product with β-chloroethylamine.
β-chloroethylamine can readily eliminate HCl, which can initiate uncontrolled polymerization of ethylenimine. Therefore, this contamination must be carefully avoided.
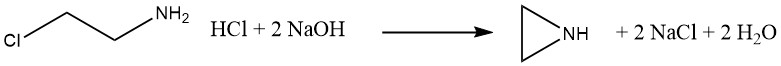
3.2. The Dow Process
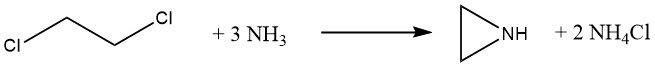
Dow Chemical developed a new method for producing ethylenimine in 1963. This method used 1,2-dichloroethane and an excess of ammonia, which promised to significantly reduce raw material costs. However, Dow discontinued production in 1978 due to the following challenges:
- Impurities in the ethylenimine product
- Elevated rates of corrosion
- Management of waste streams
3.3. The Wenker Process
The Wenker process, developed in 1935, is the basis for most commercial aziridine production today. BASF used the Wenker process for over 30 years without any technical problems.
The Wenker process has two steps. First, 2-aminoethanol reacts with sulfuric acid to produce 2-aminoethyl hydrogensulfate:

This product is a water-soluble, nonvolatile solid with properties similar to an amino acid.
This ensures that ethylenimine remains uncontaminated, unlike in the β-chloroethylamine and Dow processes. Second, 2-aminoethyl hydrogensulfate reacts with aqueous sodium hydroxide to produce ethylenimine. The cyclization is preferably carried out under pressure at elevated temperatures.
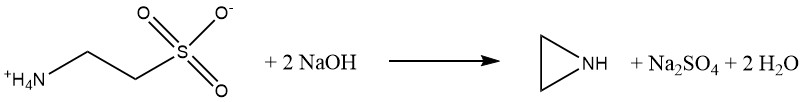
Initially, yields were only 26%, but today, yields of 85-90% are achieved. The key advantage of this process is the production of pure ethylenimine with minimal waste disposal issues. However, the downside is the relatively high cost of raw materials.
3.4. Catalytic Dehydration of 2-Aminoethanol
Researchers have been trying to develop a direct industrial synthesis for aziridine from 2-aminoethanol since the 1970s. The key to this was finding a suitable catalyst, which took many years. In 1990, Nippon Shokubai developed an industrial ethylenimine production process via catalytic dehydration of 2-aminoethanol.
The reaction is carried out at 350-450 °C, preferably under reduced pressure, using a catalyst with weakly acidic and weakly basic centers, such as SiO2 doped with barium, cesium, and phosphorus. In this gas-phase reaction, selectivities up to 90% at conversions from 40-80% can be obtained. Unconverted 2-aminoethanol is recycled from the product mixture and used as feedstock.
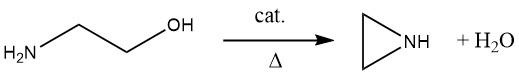
The advantage of the gas-phase process over other industrial processes is that it is a salt-free, direct one-step synthesis based on 2-aminoethanol. However, there are some disadvantages:
- Multistage distillation is required to separate ethylenimine from byproducts and recycle 2-aminoethanol.
- The process requires a lot of energy.
- The catalyst needs to be regenerated repeatedly due to coke deposition and loss of phosphorus compounds.
4. Uses of Aziridines
Aziridines are used in a variety of industries, including coatings, paper manufacturing, water treatment, petroleum, textiles, metal surface treatment, air purification, and oil and gas.
Coatings: Polyfunctional aziridines are used to cross-link polymers with carboxyl groups, creating cross-linked polymers used in performance coatings, such as those applied to exterior wood panels.
Polyethylenimines are used as tie-coat adhesives and adhesion promoters in the production of laminated films made of materials like polypropylene. They are also effective pigment dispersants and primers, particularly for acrylate-based adhesives. Additionally, polyethylenimines are used as amine components in epoxy and polyurethane resins.
Paper manufacturing: Polyethylenimine and aziridine-modified polymers are used as retention and drainage aids, and their role has grown in importance due to increased paper recycling and closed water circuits within the industry. Polyethylenimines are also used as fixing agents for soluble and insoluble contaminants, commonly referred to as “stickies.”
Water treatment: Polyethylenimines play a crucial role in water treatment as clarifying aids and effective silica antiscaling agents. They can function as chelating agents for various heavy metal ions, including copper, rhodium, mercury, and zinc.
Through carboxymethylation, their complexing capabilities can be extended to other heavy metals and alkaline earth metals. Polyethylenimines are used in heavy metal enrichment processes, often employing membrane technology.
Textiles: Polyethylenimines are used in dyeing pretreatment and posttreatment to enhance dye fixation and colorfastness. They also improve the anti-static properties of hydrophobic fibers and contribute to shrink-proofing.
Metal surface treatment: Polyethylenimines are used as brightener components in galvanic baths for metals like zinc, tin, copper, and various alloys. They are also involved in passivation and finishing processes.
Air purification: Polyethylenimines function as adsorbents for acidic gases, ozone, and aldehydes.
Oil and gas: Polyethylenimines are integral in the drilling, completion, and oil production processes in the oil and gas industry. They are combined with sulfonated polymers to inhibit fluid loss from well cement, and they serve as selective flocculants in drilling fluid and demulsifiers for certain crude oil emulsions.
Other applications: An emerging technology involves using polyethylenimine to immobilize enzymes, a process with various applications. The strong affinity of polyethylenimine for proteins, such as those found in hair or skin, opens doors to cosmetic and personal care applications.
The original high charge density of polyethylenimines can be adjusted to the desired level by chemical modifications, such as alkoxylation. Lastly, polyethylenimines are used as additives in detergents.
5. Toxicology of Aziridine
Ethylenimine is a highly toxic chemical that can be harmful to humans and animals through all routes of exposure. For example, the LD50 (oral, rat) is as low as 14 mg/kg, the LD50 (skin, rabbit) is 13 mg/kg, and the LC50 (10-minute inhalation, mouse) is 2236 ppm.
Inhalation: Exposure to high concentrations of ethylenimine can cause immediate irritation of the nose and eyes, followed by edema and lung injury. Death can occur within a few days.
Skin contact: Ethylenimine is a skin sensitizer and can cause severe irritation. It can also be absorbed through the skin and cause damage to the central nervous system, liver, and kidneys. Two deaths have been reported from inhalation and skin contact with ethylenimine.
Ingestion: Ethylenimine is also highly toxic if ingested.
Eye contact: Ethylenimine is a severe eye irritant.
Mutagenicity and carcinogenicity: Ethylenimine has been shown to be mutagenic in bacteria and fruit flies. It has also been shown to be carcinogenic in mice, but there is no evidence of carcinogenicity in humans.
Regulation: Ethylenimine is classified as a cancer suspect agent by OSHA and is subject to strict regulations to prevent exposure. The ACGIH recommends a threshold limit value (TLV) of 0.5 ppm and emphasizes the importance of avoiding skin contact.
The DFG classifies ethylenimine as an A 2 carcinogen and a category 2 germ cell mutagen, which requires special protective measures and ongoing surveillance.
Reference
- Aziridines; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a03_239.pub2
