Acetylation

In chemistry, acetylation is an organic reaction in which an acetyl group (CH3CO-) is introduced into a molecule. This reaction typically involves the use of acetic acid (CH3COOH) or acetic anhydride (CH3CO)2O as the acetylating agent. The resulting product is called acetate.
In biology, acetylation is a type of post-translational modification that involves the addition of an acetyl group to a protein. This modification can alter the protein’s activity, stability, or interactions with other molecules. Acetylation is a reversible process that is regulated by enzymes called histone acetyltransferases (HATs) and histone deacetylases (HDACs).
In this article, we focus only on acetylation in chemistry.
1. Acetylation Reaction Mechanism
The specific mechanism of acetylation can vary depending on the reagents involved and the substrates being modified.
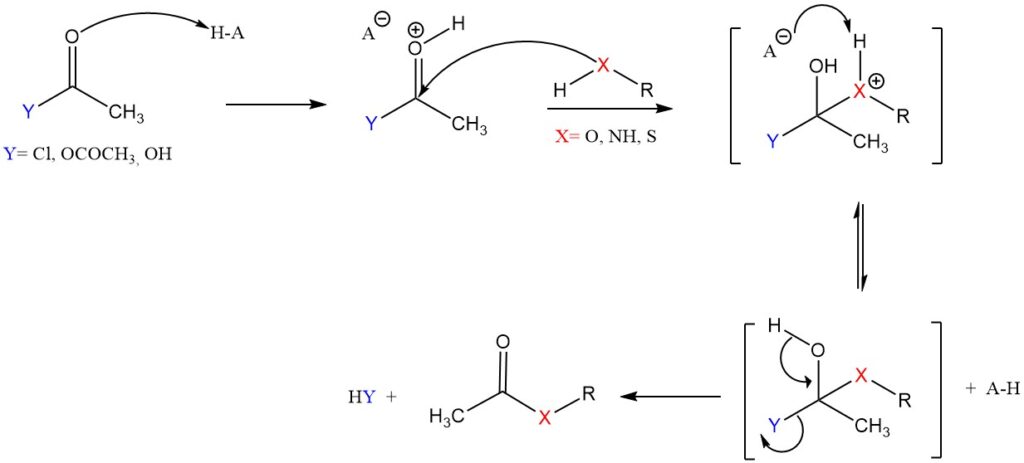
The nucleophilic acyl substitution is commonly used in organic chemistry, where an acetyl group from an acetylating agent (like acetic anhydride or acetyl chloride) replaces a hydrogen atom on the target molecule.
- The acetylating agent reacts with a homogeneous or heterogeneous acid catalyst to generate a more reactive intermediate, a cation.
- This cation, an electrophile, will be attacked by the nucleophilic atom (O, N, or S) of the substrate to form a tetrahedral intermediate.
- The leaving group (chloride or acetate) departs to form the final acetylated product and regenerate the acid catalyst.
Temperature, pH, and the presence of catalysts can significantly impact the rate and efficiency of the acetylation process. Additionally, the chemical properties and spatial arrangement of the target molecule can influence the accessibility of the site for acetylation.
2. Acetylation Agents
Common acetylation reagents used in organic and industrial chemistry are acetic anhydride, acetyl chloride, and acetic acid.
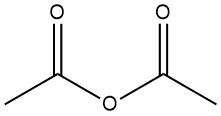
Acetic anhydride is the most commonly used reagent for acetylation in the laboratory and in the pharmaceutical industry. It is relatively inexpensive and easy to handle. However, it has the disadvantage of generating acetic acid as a byproduct, which can sometimes interfere with the reaction.

Acetyl chloride is another common reagent for acetylation. It is more reactive than acetic anhydride, but it is also more corrosive and can generate hydrogen chloride gas as a byproduct, which is harmful and corrosive for equipment.

Acetic acid is inexpensive and widely available and can also be used as an acetylation agent, but it gives a low yield and needs a dehydration catalyst to minimise the water produced as a byproduct, which favors the reversible reaction (deacetylation).
The choice of the best acetylation reagent for a particular reaction will depend on several factors, including the substrate, the desired selectivity, and the cost.
3. Examples of Important Acetylation Reactions
Aspirin (acetylsalicylic acid) is a well-known pain reliever and anti-inflammatory drug that is produced by the acetylation of salicylic acid.
Acetaminophen (N-Acetyl-p-aminophenol) is another common pain reliever and anti-inflammatory drug, often used for children and people with sensitive stomachs. It is obtained by N-acetylation of p-aminophenol.
Cellulose acetate is a type of synthetic fiber used in clothing, furniture upholstery, and cigarette filters. It is made by the acetylation of cellulose.
Acetylated monoglycerides are used as food emulsifiers and stabilizers in bread, ice cream, and other processed foods.
Ethyl acetate is a common solvent produced by the acetylation of ethanol and used in paints, coatings, and adhesives.
N-Acetylcysteine (NAC), formed by N-acetylation of cysteine, is an antioxidant supplement sometimes used to treat respiratory problems and other conditions.
3.1. Acetylation of Aniline
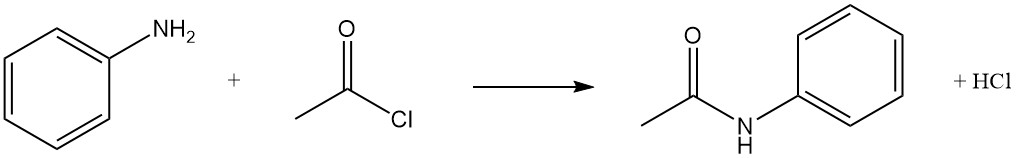
The acetylation of aniline is a fundamental reaction in organic chemistry, transforming a basic amine into a valuable intermediate with various industrial and pharmaceutical applications.
In this reaction, aniline is acetylated by acetic anhydride or acetyl chloride to produce acetanilide, often used as an intermediate in dye and pharmaceutical synthesis. Depending on the chosen acetylating agent, acetic acid or hydrogen chloride are formed as byproducts.
3.2. Acetylation of Salicylic Acid

The acetylation of salicylic acid, also known as the synthesis of aspirin, is a classic example of organic chemistry transforming a simple molecule into a pharmaceutical agent.
Salicylic acid (C₇H₆O₃) is a naturally occurring compound found in willow bark and other plants. It has pain-relieving and anti-inflammatory properties. However, it can also irritate the stomach.
Acetylsalicylic acid, which possesses enhanced pain-relieving and anti-inflammatory effects with reduced stomach irritation compared to salicylic acid, is produced by acetylation of salicylic acid with acetic anhydride in an acidic medium (e.g., concentrated sulfuric acid). Acetic acid is a byproduct of the reaction.
3.3. Amino Acid Acetylation
Amino acid acetylation is a dynamic and widespread post-translational modification (PTM) that plays a crucial role in regulating protein function, stability, and interaction with other molecules. It involves the addition of an acetyl group to specific amino acid residues, most commonly the N-terminal α-amino group or the ε-amino group of lysine residues.
This simple modification can have profound effects on protein behavior, influencing diverse cellular processes such as:
- Gene expression: Histone acetylation, where acetyl groups are added to the histone proteins packaging DNA, affects chromatin structure and accessibility, thereby regulating gene transcription.
- Enzyme activity: Acetylation can activate or deactivate enzymes by altering their structure, substrate binding, or interaction with other regulatory proteins.
- Protein-protein interactions: By affecting protein surface charges and conformation, acetylation can modulate how proteins interact with each other, impacting signaling pathways and cellular responses.
- Protein degradation: Acetylation can influence the recognition of proteins by degradation machinery, marking them for destruction or protecting them from breakdown.
There are two main types of amino acid acetylation:
- N-terminal α-acetylation: This is the most common type, occurring on the N-terminal α-amino group of newly synthesized proteins in eukaryotes. It facilitates protein folding and stability, and often serves as a signal for protein sorting and transport.
- Lysine ε-acetylation: This type involves the addition of an acetyl group to the side chain of lysine residues within the protein sequence. It can occur both co-translationally (during protein synthesis) and post-translationally (after protein synthesis). This dynamic modification plays a diverse range of regulatory roles, depending on the specific protein and site of acetylation.
