Fluorosulfuric Acid: Properties, Production and Uses
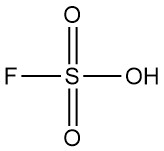
What is Fluorosulfuric Acid?
Fluorosulfuric acid is an inorganic compound with the chemical formula HSO3F. It is a colorless to pale yellow liquid with a stinging odor that fumes readily in moist air. It is recognized as one of the strongest acids commercially available.
The fluorine-sulfur bond is stronger than that of chlorosulfuric acid, so as a result, fluorosulfuric acid is hydrolyzed more slowly than chlorosulfuric acid. Under optimal conditions, the synthesis of fluorosulfuric acid salts in an aqueous solution is possible.
Fluorosulfuric acid was initially mentioned in 1892, along with a synthetic method that uses hydrogen fluoride and sulfur trioxide, which remains the preferred production process.
Historically, fluorosulfuric acid was used in fluorination reactions and as a catalyst in alkylation and cyclization processes. Industrial processes such as boron trifluoride production and tetrahydrofuran polymerization use fluorosulfuric acid. Also, it has been employed in the chemical polishing of lead crystal glass since the 1960s.
Commercial fluorosulfuric acid is composed of approximately 99.0% HSO3F, with minor impurities including sulfuric acid, sulfur trioxide, sulfur dioxide, and iron.
To date, salts and derivatives of fluorosulfuric acid have limited industrial importance.
Table of Contents
1. Physical Properties of Fluorosulfuric Acid
Fluorosulfuric acid is a colorless liquid that is soluble in polar organic solvents (e.g., nitrobenzene, acetic acid, and ethyl acetate) but poorly soluble in nonpolar solvents.
The physical properties of fluorosulfuric acid are summarized in Table 1.
| Property | Value |
|---|---|
| CAS number | [7789-21-1] |
| Chemical formula | HSO3F |
| Molecular weight | 100.07 g/mol |
| Melting point | - 88.98 °C |
| Boiling point |
at 101.3 kPa: 162.7 °C at 16.0 kPa: 110 °C at 2.5 kPa: 77 °C |
| Density |
at 18 °C: 1.740 g/cm3 at 25 °C: 1.725 g/cm3 |
| Vapor pressure at 25 °C | 330 Pa |
| Viscosity at 25 °C | 1.56 mPa.s |
| Heat of formation (liquid) | 792.45 kJ/mol |
2. Production of Fluorosulfuric Acid
Fluorosulfuric acid is industrially produced by stirring hydrogen fluoride and sulfur trioxide in fluorosulfuric acid as a solvent for the reaction. The fluorosulfuric acid is cooled to keep the temperature in the reactor constant at < 100 °C, as shown in Figure 1.
HF + SO3 → HSO3F
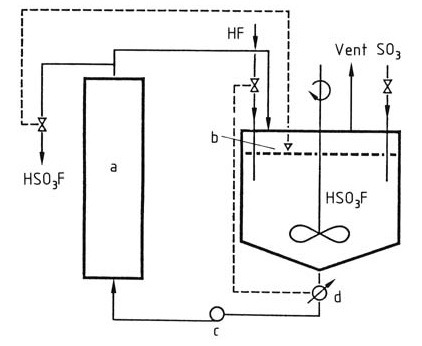
Figure 1: Production of Fluorosulfuric Acid
a) Cooler; b) Level control; c) Pump; d) Conductometer
Alternatively, fluorosulfuric acid can be synthesized by reacting potassium hydrogen fluoride or calcium fluoride with oleum (fuming sulfuric acid) at elevated temperatures (around 250 °C).
After the reaction, any remaining hydrogen fluoride is removed by purging with an inert gas. The resulting fluorosulfuric acid can then be purified by distillation in a glass apparatus.
Due to the corrosive nature of fluorosulfuric acid, its production and handling require specialized equipment and rigorous safety protocols.
3. Uses of Fluorosulfuric Acid
3.1. Uses of Fluorosulfuric Acid in Inorganic Chemistry
Fluorosulfuric acid is used as a fluorinating agent that exhibits milder reactivity compared to hydrofluoric acid. Examples of fluorination reactions include the conversion of silicon dioxide to silicon tetrafluoride, boric acid to boron trifluoride, arsenic trioxide to arsenic trifluoride, and potassium perchlorate to perchloryl fluoride. The latter compound is employed as a fluorinating agent in organic synthesis.
3.2. Uses of Fluorosulfuric Acid in Glass Polishing
Fluorosulfuric acid is used to restore lead glass polishing baths, which comprise 60–70% sulfuric acid, 2-6% hydrofluoric acid, and water. The polishing process consumes these acids by partially dissolving the glass surface, as shown in the following equations:
PbO + H2SO4 → PbSO4 + H2O
SiO2 + 6 HF → H2SiF6 + 2 H2O
When fluorosulfuric acid is added to the polishing bath, it is hydrolyzed to reproduce these acids in the acid medium by consuming some of the formed water.
HSO3F + H2O → H2SO4 + HF
This method reduces hydrofluoric acid consumption by approximately 28% and also reduces waste acid compared to traditional regeneration techniques using sulfuric and hydrofluoric acids.
3.3. Uses of Fluorosulfuric Acid in Organic Chemistry
Fluorosulfuric acid catalyzes alkylation, polymerization, and isomerization reactions in organic synthesis. The reactive alkyl fluorosulfate intermediate is responsible for the catalytic activity. Examples of this reaction include:
- Alkylation of olefins and isoparaffins
- Formation of high-octane fuels from low-octane hydrocarbons
- Production of alkylated aromatics and alkylation of phenol to tert-butylphenol.
Additionally, it is used in polymerization reactions, such as the polymerization of tetrahydrofuran, of ethylene to long-chain unsaturated hydrocarbons, and of trioxane to polyoxymethylene. Isomerization of C7 hydrocarbons, pentene, and methylpentene is also catalyzed by fluorosulfuric acid.
Selective production of beta-naphthol from naphthalene by catalytic hydroxylation with hydrogen peroxide is achieved using a combination of fluorosulfuric acid and antimony pentafluoride as a catalyst system.
3.4. Uses of Fluorosulfuric Acid in Fluorine Purification
Fluorosulfuric acid offers an alternative to sodium fluoride for removing hydrogen fluoride from elemental fluorine. Unlike sodium fluoride, which forms sparingly soluble sodium hydrogen fluoride, the product formed with fluorosulfuric acid can be removed by distillation.
4. Safety Hazards and Handling of Fluorosulfuric Acid
Fluorosulfuric acid poses significant risks to human health due to its corrosive nature and reactivity. Contact with eyes, skin, or mucous membranes can result in severe burns and tissue damage. Exposure to vapors can irritate respiratory tract. Hydrolysis of fluorosulfuric acid in the presence of moisture generates hydrogen fluoride and sulfuric acid, exacerbating the corrosive effects.
Hydrogen fluoride has unique toxicological properties; it penetrates skin and causes deep tissue damage. Its ability to chelate calcium and magnesium ions disrupts cellular metabolism.
Strict adherence to safety protocols is imperative when handling fluorosulfuric acid. Personal protective equipment, including respiratory protection, chemical-resistant gloves, eye protection, and full body suits in severe cases, is mandatory.
Emergency procedures for exposure incidents require immediate decontamination with water and a calcium gluconate solution, followed by medical attention. Ingestion requires administration of milk of magnesia or calcium gluconate solution.
Fluorosulfuric acid can ignite organic materials under specific conditions. Spills must be handled with extreme caution, using large quantities of water for dilution while preventing the formation of corrosive mists.
The addition of cold concentrated sulfuric acid, followed by dilution and neutralization with lime, is recommended for small spills.
Reference
- Fluorosulfuric Acid; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a11_431.pub2
