Cyanuric Chloride: Properties, Reactions, Production and Uses
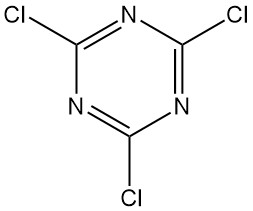
What is Cyanuric Chloride?
Cyanuric chloride, also known as 2,4,6-trichloro-1,3,5-triazine, is an organic compound with the formula C3N3Cl3. It appears as a colorless, crystalline solid with a pungent odor. It is the chlorinated derivative of 1,3,5-triazine and the trimer of cyanogen chloride.
Table of Contents
1. Physical Properties of Cyanuric Chloride
Cyanuric chloride forms colorless monoclinic crystals of pungent odor, reminiscent of acetamide and acid chlorides. It is soluble in most organic solvents but insoluble in water.
Table 1 shows the solubility (in wt%) of cyanuric chloride in various solvents at 25 °C.
| Solvent | Solubility (wt %) |
|---|---|
| Acetone | 25.0 |
| Acetonitrile | 21.0 |
| Acrylonitrile | 19.0 |
| Benzene | 19.0 |
| Chlorobenzene | 16.0 |
| Tetrachloromethane | 7.5 |
| Diethyl ether | 14.0 |
| Dioxane | 55.0 |
| Ethyl acetate | 21.0 |
| Methyl vinyl ketone | 27.0 |
| Nitrobenzene | 18.0 |
| Tetrahydrofuran | 43.0 |
| Trichloromethane | 20.0 |
| Toluene | 18.5 |
Cyanuric chloride sublimes before reaching the boiling point. The vapor pressure of the liquid cyanuric chloride as a function of temperature is given by the following equation:
ln p (mmHg) = -0.33480-742.7311/T(K)+11.15147 ln T(K)
Important physical properties of cyanuric chloride are listed in the following table.
| Property | Value |
|---|---|
| CAS number | [108-77-0] |
| Formula | C3N3Cl3 |
| Molecular weight | 184.5 g/mol |
| Triple point | 145.7 °C at 255 kPa |
| Boiling point | 194 °C |
| Density (solid) | 1.92 g/cm3 |
| Density (melt) | 1.48 g/cm3 |
| Heat capacity | 0.99 kJ kg-1 K-1 at 150 °C |
| Heat of fusion | 123 kJ/kg |
| Heat of vaporization | 256 kJ/kg |
2. Reactions of Cyanuric Chloride
Under anhydrous conditions, cyanuric chloride acts as a chlorinating agent. Alcohols and tertiary amines are converted to alkyl chlorides, while carboxylic acids in anhydrous acetone with triethylamine form acid chlorides, which are isolated or used in situ for the production of esters and amides. Cyanuric acid is a byproduct in both cases.
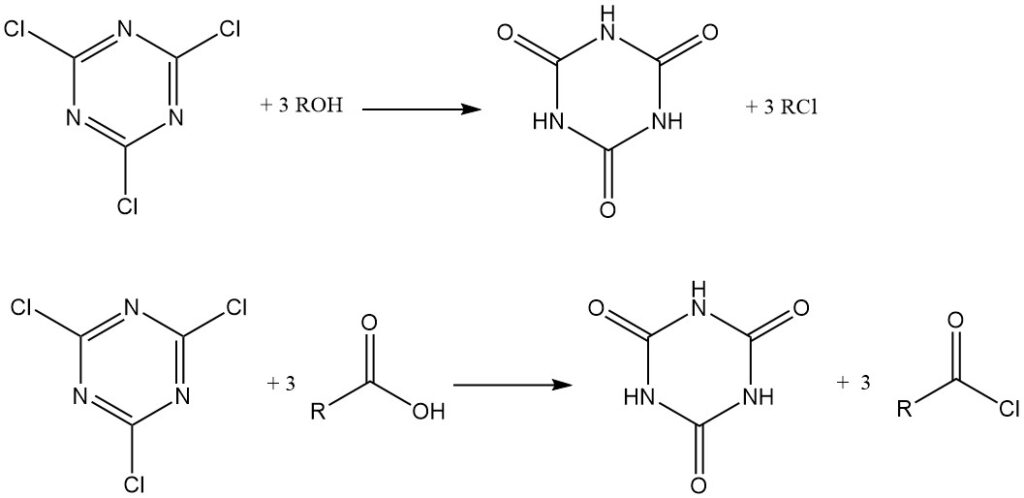
It can also be used as a condensation (dehydrating) reagent. Amides, thioamides, and aldoximes can be converted to nitriles.
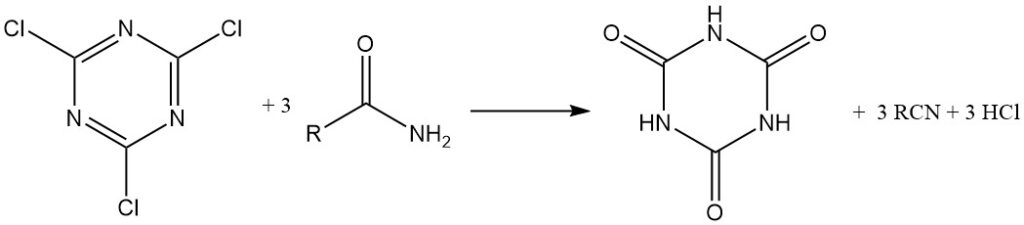
Under anhydrous conditions, tertiary aliphatic amines react with cyanuric chloride to form substituted melamine derivatives, with one alkyl group converted to the corresponding alkyl chloride.
Cyanuric chloride typically reacts like acid chloride, forming hydrogen chloride. While a suspension in ice water exhibits some stability (up to 12 hours at 0 °C), hydrolysis is accelerated when the temperature increases, with over 40% hydrolyzed within 1 hour at 30 °C. Cyanuric acid is the final hydrolysis product.
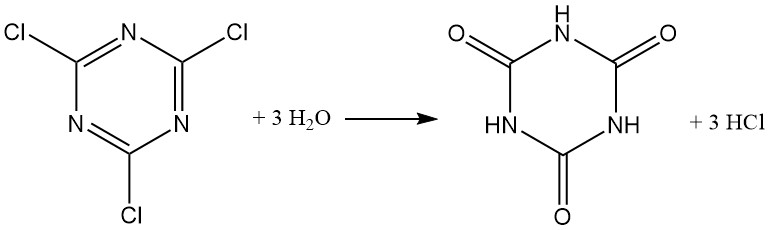
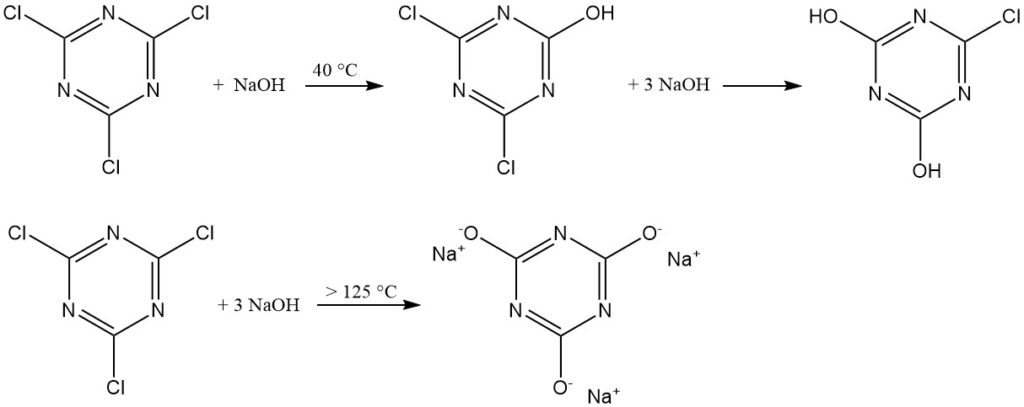
Alkaline aqueous mediums (NaOH or NaHCO3 at 40 °C) promote the formation of 2,4-dichloro-6-hydroxy-1,3,5-triazine. Excess base yields 2,4-dihydroxy-6-chloro-1,3,5-triazine. Trisodium cyanurate formation only occurs above 125 °C.
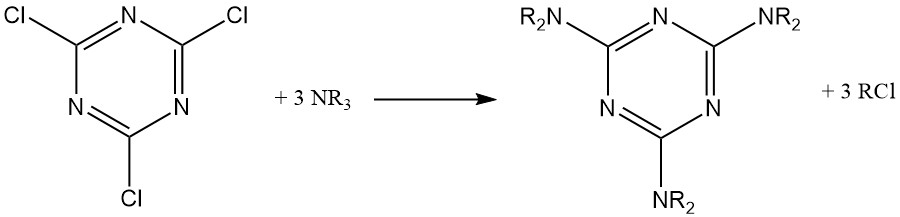
Cyanuric chloride’s temporary stability in water allows reactions with strong nucleophiles (such as alcohols, thiols, and primary and secondary amines) in ice water suspensions. These reactions often proceed step-by-step to achieve trisubstitution at controlled temperatures.
An empirical rule suggests replacing the first chlorine at 0–5 °C, the second at 30–50 °C, and the third at 70–100 °C for amine substituents. HCl scavengers include sodium hydroxide, sodium hydrogencarbonate, disodium hydrogenphosphate, and tertiary amines.
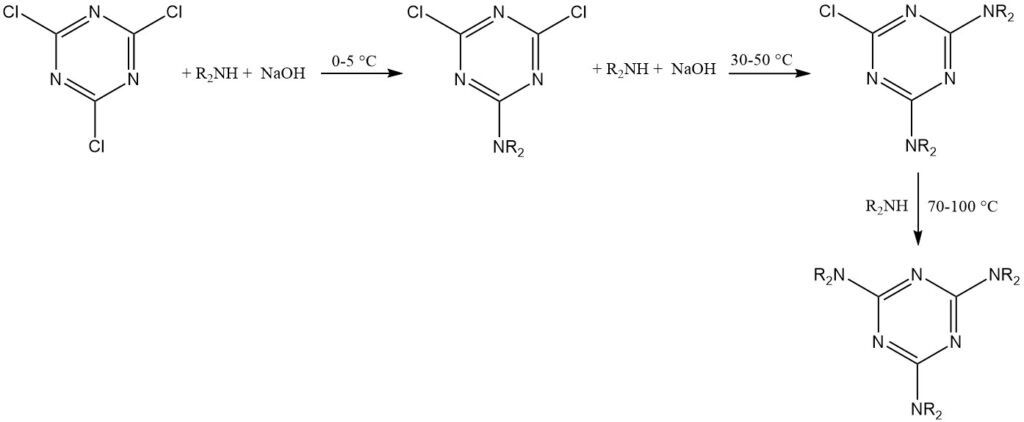
The triazine product is typically isolated by filtration or centrifugation. Acetone, methyl ethyl ketone, or toluene are common solvents for these reactions.
Cyanuric chloride (melt or powder) is dissolved/suspended in the solvent with the nucleophile and then treated with aqueous NaOH. The product is isolated from the organic layer by evaporation or filtration.
This method allows for the sequential introduction of various substituents at different temperatures. High-boiling solvents or the molten reactant might be necessary to replace the third chlorine atom.
The neutralization of liberated hydrochloric acid and heat dissipation during reactions are crucial. Exothermic hydrolysis of cyanuric chloride (ΔH = -2164 kJ/kg) can become uncontrollable, especially in cyanurate production from alcohols.
Water-miscible solvents accelerate hydrolysis. Storing cyanuric chloride solutions in aqueous acetone without proper heat removal can lead to runaway reactions even at room temperature.
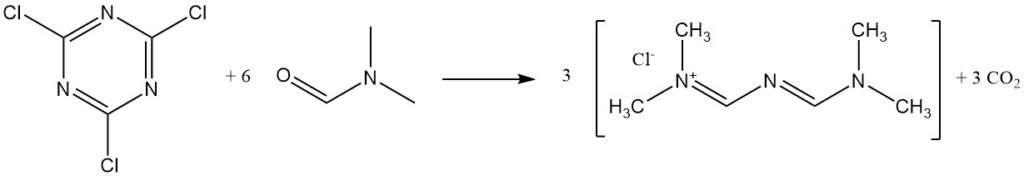
Cyanuric chloride reacts with DMF to form Gold’s salt.
3. Production of Cyanuric Chloride
Cyanuric chloride production facilities are typically located near hydrocyanic acid (HCN) plants, the primary raw material. Sodium cyanide can also be used, but to a lesser extent.
The production process of cyanuric chloride involves two main steps: chlorination of HCN to cyanogen chloride (ClCN) and trimerization of ClCN to cyanuric chloride.
First, HCN reacts with chlorine (Cl2) in a loop reactor at moderate temperatures between 20 and 40 °C to form ClCN. This reaction is exothermic (ΔH = -89 kJ/mol).
Then, the ClCN is separated, purified, and then trimerized (combining three molecules into one) on activated carbon at temperatures exceeding 300 °C. This step is also exothermic (ΔH = -233 kJ/mol).
Figure 1 illustrates a detailed process for the production of cyanuric chloride from chlorine and hydrocyanic acid.
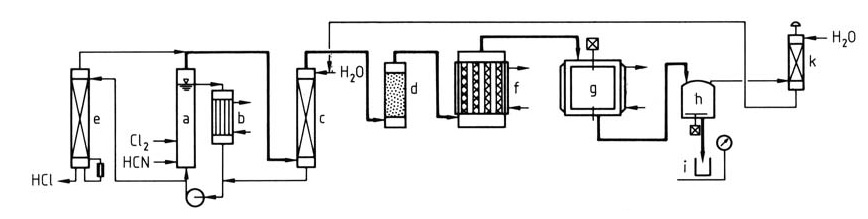
a) ClCN reactor; b) Heat exchanger; c) Scrubber; d) Dryer; e) Effluent stripper; f) Trimerizer; g) Condenser; h) Storage; i) Drum; k) Tail gas scrubber
A mixture of HCN and Cl2 is fed into the loop reactor (a & b). The saturated aqueous solution of ClCN exits the loop, then the ClCN is washed with water (c) to remove impurities and dried (d).
The wash water containing HCl is sent to a stripper (e) to recover the dissolved ClCN and release concentrated HCl for further use. Alternatively, sections (e), (a), and (c) can be combined into a single unit. The purified, dry ClCN undergoes trimerization (f) on activated carbon to form cyanuric chloride vapors.
The vapors are condensed into molten or solid cyanuric chloride (g). The product can be dissolved in a solvent for captive use or packaged in containers (h & i). Tail gases are scrubbed and recycled (k) to minimize waste.
This process typically achieves ClCN yields exceeding 95% and cyanuric chloride yields exceeding 90%.
Several variations aim to eliminate dilute HCl formation during ClCN production. These include one-step gas-phase reactions using charcoal, tetrachloroethane, or cyanogen and chlorine on charcoal.
Other catalysts for ClCN trimerization, like fused cyanuric chloride, metal chlorides, eutectic mixtures, and zeolites, have been explored.
In labs, ClCN solutions in organic solvents such as benzene and chloroform can be trimerized using HCl. Commercial processes might use the hydrogen chloride-dimethyl ether azeotrope for trimerization.
Equipment for ClCN production uses materials resistant to corrosion, such as glass, fluoropolymers, graphite, or special resins. Cyanuric chloride production equipment typically uses nickel, stainless steels, or aluminum.
Moisture is a significant concern in this process and can cause severe corrosion. Proper equipment, materials, and handling procedures are important to reduce this risk.
4. Uses of Cyanuric Chloride
Cyanuric chloride is used as a precursor for pesticides and herbicides, in the production of dyes, brighteners, and UV light stabilizers in plastics, and in organic synthesis.
The most significant cyanuric chloride derivatives are aminotriazines, particularly alkylaminotriazines, which are used as pesticides and herbicides. Triazine-based herbicides remain one of the most commercially successful, with most formulations containing 2-chloro- or 2-methylthio-4,6-dialkylamino-1,3,5-triazines.
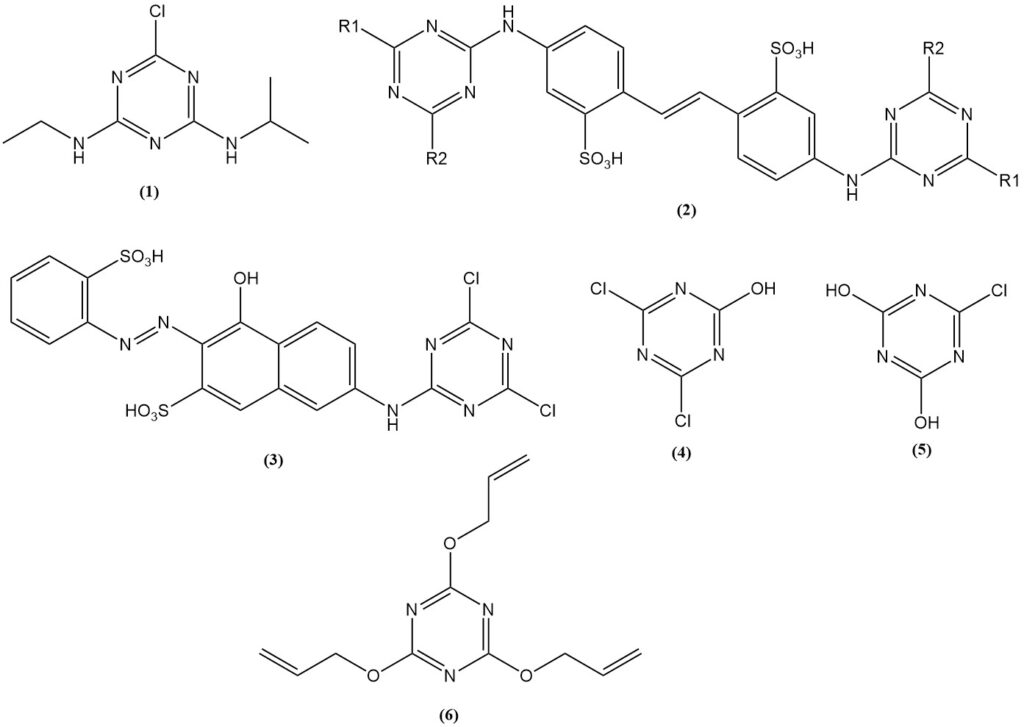
Atrazine (1) is the most important triazine herbicide, accounting for over 70% of cyanuric chloride consumption dedicated to herbicide production. Triazine herbicides are manufactured globally.
Less than 30% of cyanuric chloride production is used in other industrial applications, such as optical brighteners, UV stabilizers, reactive dyes, and cross-linking agents.
Reaction products of aminostilbenes with substituted triazines, particularly bis(triazinylamino)stilbenedisulfonic acids (2), are used as brightening agents for fabrics and paper.
Dye derivatives prepared from cyanuric chloride can react chemically with fabrics. The Procion dyes (3) were among the first.
Cyanuric chloride and cyanurates are used in the production of gelatin and glues. Examples include sodium salts of 2,4-dichloro-6-hydroxy-1,3,5-triazine (4) and 2,4-dihydroxy-6-chloro-1,3,5-triazine (5). Also, triallyl cyanurate (6) is used in the rubber and plastics industries.
Triazines have various other uses, including as modifiers, accelerators, flame retardants, pharmaceuticals, antioxidants, antiozonants, and heavy metal scavengers.
5. Toxicology of Cyanuric Chloride
Cyanuric chloride poses a severe irritant to the skin, mucous membranes (including the eyes), respiratory tract, and gastrointestinal tract. Its 1-minute irritation threshold for mucous membranes is exceptionally low at 0.3 mg/m³. Allergic reactions can also occur.
Direct contact with cyanuric chloride must be strictly avoided. Ensure proper ventilation in work environments.
When handling cyanuric chloride vapor or dust, wear a full-face gas mask equipped with an active carbon canister or use a self-contained breathing apparatus.
Cyanuric chloride is labeled with the hazard symbol Xi and assigned to risk phrases (R phrases) R 36/37/38, indicating irritation to the eyes, respiratory system, and skin. Additionally, safety phrases (S) 28 emphasize the need for wearing suitable protective clothing.
The following R and S phrases should also be applied for comprehensive safety labeling:
- R 22: Harmful if swallowed
- R 26: Very toxic by inhalation
- R 41/43: May cause sensitization by inhalation and skin contact
- S 36/37/39: Wear suitable protective clothing, gloves, and eye/face protection
- S 45: In case of accident or if you feel unwell, seek medical advice immediately
When adding cyanuric chloride powder to flammable solvents, use grounded funnels to prevent electrostatic discharge. Only qualified personnel trained in proper handling procedures should be authorized to work with cyanuric chloride.
Reference
- Cyanuric Acid and Cyanuric Chloride; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a08_191
