Triethylamine: Properties, Production and Uses
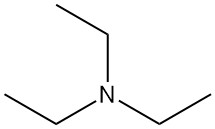
Triethylamine (TEA) is tertiary amine, represented by the chemical formula N(CH2CH3)3. It is a colorless easily flammable liquid with an ammonia-like odor. It is one of the most widely used organic amine base in synthetic organic chemistry.
Table of Contents
1. Physical Properties of Triethylamine
Triethylamine is a colourless, unpleasant-smelling, inflammable liquid. Important physical properties are listed in the following table:
| Property | Value |
|---|---|
| CAS registry number | [121-44-8] |
| Chemical formula | (C2H5)3N |
| Color | Colorless |
| Physical state | Liquid |
| Molecular weight (Mr) | 101.19 |
| Melting point (mp), °C | -115 |
| Boiling point (bp), °C | 89.3 |
| Density (d) at 20 °C | 0.7275 |
| Refractive index (n) at 20 °C | 1.4010 |
| Flash point, °C | -11 |
| pKb (25 °C) | 3.24 |
| Dissociation constant (pKa) | 10.76 |
| Odor threshold, ppm; v/v | 0.48 |
| Solubility in Water | Miscible in water (112 g/L at 20 °C) |
| Solubility in Organic solvents | Miscible in ethanol and ethyl ether |
| Vapor pressure density | 3.48 |
| Vapor pressure at 20 °C, torr | 54 |
| Auto flammability (°C) | 215 |
2. Chemical Reactions of Triethylamine
Triethylamine presents high fire and safety hazards when exposed to heat, flame, or oxidizers, and it becomes explosive in vapor form.
Undiluted triethylamine complexed with dinitrogen tetraoxide can explosively decompose below 0 °C.
Triethylamine reacts exothermically with maleic anhydride above 150 °C, generating heat and potentially posing fire hazards. Upon decomposition due to heat, it emits toxic nitrogen oxide fumes (NOx).
The main reactions of triethylamine are:
1. Formation of salts with acids
Due to its alkyl substituents, triethylamine is a stronger base than ammonia, forming highly water-soluble salts with organic and inorganic acids. The difference in solubility between triethylamine and its salt makes it a good acid acceptor and solvent for gas scrubbing and for certain extraction processes like semisynthetic penicillin production.
Also, it acts as a potent proton acceptor in various reactions, including oxidations, reductions, eliminations, substitutions, and additions. The resulting quaternary salts are readily removed after completion.
2. Alkylation
The reaction of triethylamine with alkyl halides and dialkyl sulfates to give, ultimately, quaternary ammonium compounds is used in preparative pharmaceutical chemistry and for the preparation of anticorrosion agents and biocides.
3. Oxidation
Triethylamine is oxidized by hydrogen peroxide to triethylamine oxide.

3. Production of Triethylamine
Triethylamine is produced by reacting ammonia with ethanol, N,N-diethylacetamide with lithium aluminum hydride, ethyl chloride with ammonia under heat and pressure. and gas-phase catalytic hydrogenation of acetonitrile.
3.1. Production of Triethylamine by reaction of Ammonia with Ethanol

The most common method involves reacting ammonia (NH3) with ethanol (CH3CH2OH) at high temperatures and pressures over a dehydration or dehydrogenation catalyst. Catalysts like alumina, silica-alumina, or silver effectively promote the reaction, yielding a mixture of ethylamine, diethylamine, and triethylamine.
Subsequent separations by extractions and distillations isolate triethylamine with high purity. This method offers high yields but necessitates careful handling of volatile intermediates and energy-intensive reaction conditions.
Mixture of mono-, di- and triethylamine can also be synthesized from acetaldehyde, ammonia, and hydrogen in the presence of a hydrogenation catalyst.

a) Vaporizer; b) Heat exchanger; c) Superheater; d) Catalytic converter; e) Product cooler; f) Gas separator; g) Ammonia column; h) Monoethylamine column; i) Diethylamine column; j) Decanter; k) Triethylamine column
3.2. Production of Triethylamine by reaction of N,N-Diethylacetamide and Lithium Aluminum Hydride

A specialized route uses N,N-diethylacetamide (CH3CH2CON(CH2CH3)2) as a substrate. Reduction with lithium aluminum hydride (LiAlH4) directly generates triethylamine. This method avoids the complex mixture of amine products but requires specialized reagents and specific safety protocols due to the pyrophoric nature of LiAlH4.
3.3. Production of Triethylamine by reaction of Ethyl Chloride and Ammonia

Under high temperature and pressure, ethyl chloride (CH3CH2Cl) can react with ammonia to form triethylamine. This process, however, typically requires additional purification steps to remove byproducts (HCl) and residual reactants.
3.4. Production of Triethylamine by Gas-Phase Catalytic Hydrogenation of Acetonitrile

Triethylamine can be produced continuously by hydrogenation of acetonitrile in the gas phase, using a noble metal from Group VIII of the periodic system (e.g., platinum, palladium) as a catalyst.
Lithium aluminum spinel support is used to enhance the catalyst performance. This reaction is operated at a temperature of 80 °C to 115 °C and a pressure of 1 to 60 bars (relative pressure).
Up to 3% by weight of monoethylamine and/or diethylamine are added relative to acetonitrile. These act as promoters, potentially improving the reaction rate or selectivity.
4. Uses of Triethylamine
The predominant use of triethylamine (TEA) is as a catalyst in the curing of resin systems within foundry molds, particularly in the production of sand cores for cold-box or isocure processes. TEA is vaporized into a gas and introduced into the system.
Apart from its role in foundry applications, TEA finds extensive use as a curing catalyst in phenol-formaldehyde particleboard adhesives, with an annual consumption of around 5 million pounds in the U.S.
Additionally, it is used in the purification of penicillin and cephalosporin antibiotics and in the interfacial polymerization process for producing polycarbonate resins.
Triethylamine is also employed as a scavenger of HCl in reactions, such as during the manufacture of benzyl phthalates, with a focus on recovery and recycling.
TEA serves as an ingredient in sealing paint (0.5% w/w), in the manufacture of paper and board adhesives, and as a stabilizer for chlorinated solvents like perchloroethylene and trichloroethylene.
It works as a catalyst in the formation of urethane foams and epoxy resins and participates in dehydrohalogenation reactions and acid neutralization for condensation reactions. TEA also finds application in reverse-phase high-performance liquid chromatography (HPLC) as a mobile-phase modifier.
In specific industries, TEA is employed as a neutralization agent for anionic stabilized waterborne resins, including polyesters, alkyds, acrylic resins, and polyurethanes containing carboxyl or other acidic groups.
Other uses of triethylamine include its use as an accelerator activator for rubber, as a corrosion inhibitor, as a propellant, as an emulsifying agent for dyes, as an ingredient in photographic development accelerators, for drying printing inks, in carpet cleaners, in the production of herbicides and pesticides, and in the preparation of emulsifiers for pesticides.
5. Toxicology of Triethylamine
1. Acute Toxicity
- Inhalation: Human volunteers exposed to triethylamine vapors experienced visual disturbances and changes in brain activity. Occupational exposure has also been linked to ocular and respiratory issues. Animal studies reveal severe toxicity and mortality following inhalation of high doses, with LD50 values ranging from 420 to 10,000 mg/m³ in rats.
- Ingestion: Oral exposure in animals resulted in dose-dependent toxicity, with LD50 values ranging from 450 to 1000 mg/kg in rodents. In humans, ingested triethylamine is primarily excreted unchanged, with a minor portion metabolized to triethylamine-oxide and diethylamine.
- Dermal: Triethylamine exhibits significant dermal irritation in animals, with LD50 values around 0.5-0.794 mL/kg in rabbits.
2. Chronic Toxicity
- Inhalation: Long-term exposure (months) to moderate triethylamine concentrations in rats caused lung, brain, and liver alterations. Nervous system changes, anemia, and chronic lung inflammation were observed at higher exposure levels.
- Oral: No adverse effects were reported in rats administered triethylamine orally for 2 months at a moderate dose. However, higher doses led to behavioral changes and convulsions, with females exhibiting greater sensitivity.
- Genotoxicity: In vitro and in vivo studies yielded conflicting results regarding triethylamine’s genotoxicity. While some assays suggested mutagenic potential in bacteria, others showed no clastogenic or aneugenic effects in rats.
3. Reproduction and Development
- Reproductive Studies: A multigenerational rat study revealed no significant impact on fertility or offspring viability, although a slight reduction in average body weight was observed in the third generation.
- Developmental Toxicity: Triethylamine administration to pregnant rabbits disrupted early embryonic development.
4. Human Epidemiology
- Cancer: A study in a Danish foundry linked occupational triethylamine exposure to an increased risk of bladder cancer mortality.
- Sensitization: Animal studies suggest low potential for skin or respiratory sensitization to triethylamine.
5. Other Observations
- Triethylamine inhibits monoamine oxidase (MAO) and sulfotransferase activity in animal tissues, potentially impacting neurotransmitter and steroid metabolism.
- In vitro studies suggest triethylamine interacts with cell protein degradation and synthesis, and may induce lysosomal swelling.
References
- Amines, Aliphatic; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_001.pub2
- Process for the preparation of triethylamine. – https://patents.google.com/patent/US4297512A/en
- Triethylamine; Review of Toxicological Literature
- Triethylamine. – https://onlinelibrary.wiley.com/doi/10.1002/3527600418.mb12144e0013
- Triethylamine anhydrous(BASF). – https://products.basf.com/global/en/ci/triethylamine-anhydrous.html
