Trichloroacetaldehyde (Chloral)
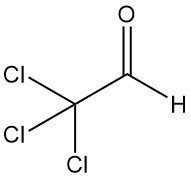
Trichloroacetaldehyde [75-87-6], also known as chloral or 2,2,2-trichloroethanal, is an organic compound with the formula CCl3CHO. It is a colorless liquid that was first produced in 1832 by Justus von Liebig through the chlorination of ethanol.
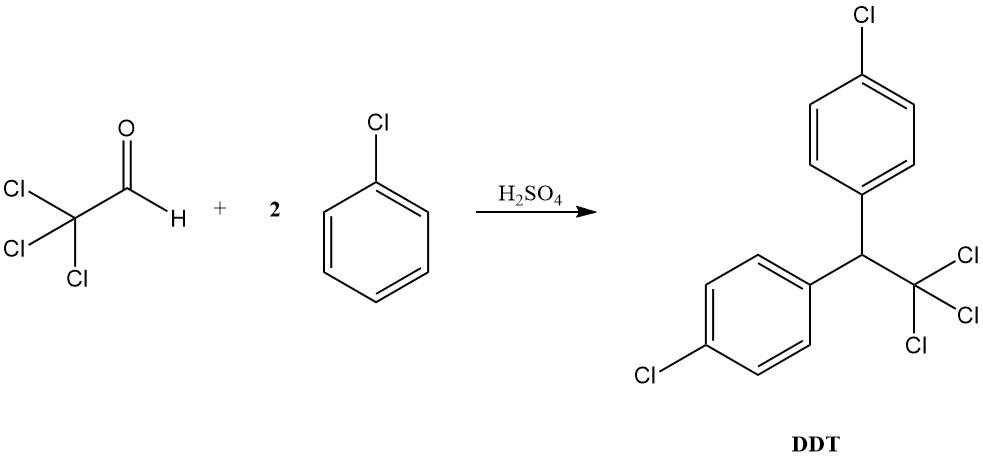
Chloral holds historical significance as the first hypnotic drug, introduced in 1869. Furthermore, it serves as a precursor to the well-known insecticide DDT (1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane), discovered in 1941.
While chloral was a valuable chemical in the 1960s, its usage has significantly declined since then because of the restrictions on DDT and other chlorinated insecticides due to environmental concerns.
Table of Contents
1. Physical Properties of Trichloroacetaldehyde
Chloral, also known as trichloroacetaldehyde or trichloroethanal, exhibits the following physical properties:
- Molecular weight: 147.39 g/mol
- Melting point: −56.5 °C
- Boiling point: 97.8 °C at 101.3 kPa
- Density: 1.5121 g/cm³ at 20 °C
- Refractive index: 1.4557 at 20 °C
- Vapor pressure: 4.7 kPa at 20 °C
- Appearance: Colorless, mobile, oily liquid
- Odor: Penetrating
- Solubility: Readily soluble in water, alcohol, ether, and chloroform
- Octanol-water partition coefficient: 0.99 (low)
- Azeotrope formation: Forms azeotropes with 1,2-dichloroethane, heptane, and benzene
2. Chemical Reactions of Trichloroacetaldehyde
Trichloroacetaldehyde has distinctive chemical properties compared to typical aldehydes, primarily due to the strong electron-withdrawing influence of its three chlorine atoms. It decomposes upon exposure to sunlight or heat.
Chloral readily reacts with nucleophiles, forming various addition products. With alcohols, it generates isolable hemiacetals (CCl3CH(OH)OR) and, with water, forms the well-known chloral hydrate [302-17-0] (CCl3CH(OH)2, mp 53 °C and bp 97.5 °C).
Unlike most aldehydes, chloral cannot participate in many base-catalyzed condensations. Strong bases tend to cleave its carbon-carbon bond, leading to products like chloroform and HCO2Na, or HCONR2 (R = alkyl), depending on the specific reagent.
CCl3CHO + NaOH → CHCl3 + HCO2Na
CCl3CHO + R2NH → CHCl3 + HCONR2 (R = alkyl)
Under acidic conditions, chloral behaves more like a typical aldehyde, forming acetals with alcohols and condensing with aromatic compounds to produce diaryltrichloroethanes, as exemplified by DDT formation from chlorobenzene.
In the presence of anionic initiators like lithium tert-butoxide, chloral polymerizes into an insoluble, nonflammable high polymer of the formula [CH(CCl3)O]n. This polymer, however, has no known commercial applications.
Oxidation of trichloroacetaldehyde yields trichloroacetic acid.
3. Production of Trichloroacetaldehyde
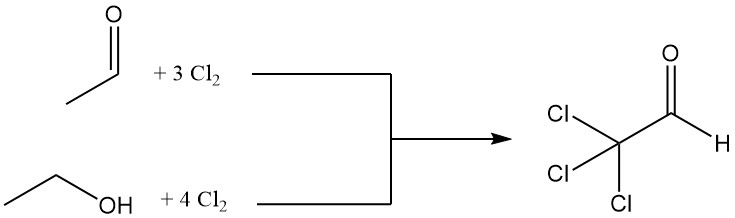
Chloral is produced by the chlorination of either acetaldehyde or ethanol, with acetaldehyde often preferred due to its economic advantages.
CH3CHO + 3 Cl2 → CCl3CHO + 3 HCl
Acetaldehyde reacts with chlorine in a hydrochloric acid solution to reduce condensation byproducts and oxidation of the aldehyde. Antimony trichloride is used as a catalyst.
The temperature gradually increases from 0 °C to 90 °C to maintain the reaction rate.
Chloral is distilled from the mixture as hydrate, which is then decomposed with concentrated sulfuric acid to separate from the heavier acid layer. It is then distilled by a fractionating column.
The chlorination of one mole of ethanol requires four moles of chlorine, with one mole used for oxidation to acetaldehyde.
CH3CH2OH + Cl2 → CH3CHO + 2 HCl
Reaction vessels and piping are lined with acid-resistant materials (ceramic, etc.) to withstand hot hydrochloric acid. Valves are made of nickel-molybdenum alloys (Hastelloy B) for corrosion resistance.
Technical-grade chloral purity ranges from 94% to 99%, with water as the main impurity.
4. Uses of Trichloroacetaldehyde
Chloral was initially used in the production of the insecticide DDT, but its uses extend far beyond that. Though DDT is banned in many countries, chloral has been used in the production of various other insecticides, like Methoxychlor and Naled, and herbicides (trichloroacetic acid).
It is also used in the production of rigid polyurethane foam.
Trichloroacetaldehyde can induce the swelling of starch granules at room temperature, potentially impacting various starch-based industries.
Chloral hydrate, the hydrate form of chloral, was used in medicine as a sedative and hypnotic drug (chloral betaine, α-chloralose, and triclofos sodium). While primarily used for short-term insomnia treatment, it has also been used to manage anxiety, induce sedation before procedures, and treat withdrawal symptoms from alcohol and other drugs.
Its use in children has declined in favor of safer alternatives, but it still finds application in certain medical situations.
In microscopy, Hoyer’s solution, containing chloral hydrate, is used to mount various organisms like bryophytes, ferns, seeds, and arthropods for detailed observation.
5. Toxicology of Chloral
Exposure symptoms:
- Chloral and its hydrate exhibit identical properties and act as central nervous system depressants.
- Therapeutic doses (0.5–1.0 g) minimally affect respiration and blood pressure, but higher doses depress both.
- Synergistic effects occur with ethanol, amplifying chloral’s action.
- Skin and mucous membrane irritation is a known side effect.
- Metabolism converts chloral to trichloroethanol, the primary contributor to its physiological effects.
- Excretion predominantly occurs through urine glucuronide conjugation.
Toxicity:
- Oral ingestion of 4–30 g has proven lethal in adults.
- Inhalation studies in rats demonstrate high toxicity, with an LC50 of 440 mg/m³ for 4-hour exposure.
- Chronic exposure at lower concentrations (75–78 mg/m³) induces severe lung injury and mortality in rats.
- Mutagenicity is observed in the Ames test.
- No established Threshold Limit Values (TLV) or Maximum Allowable Concentrations (MAK) exist for chloral, prompting cautious handling similar to chloroacetaldehyde.
Carcinogenicity:
- The International Agency for Research on Cancer (IARC) classifies chloral and chloral hydrate as Group 3: “not classifiable as to its carcinogenicity to humans”.
- While limited evidence suggests carcinogenicity in animal studies, human data remains inadequate.
EU Classification:
- Based on Directive 67/548/EEC, chloral and chloral hydrate are designated as “Toxic” (T) with risk phrases R25-36/38, signifying toxicity upon ingestion and skin/eye irritation.
Reference
- Chloroacetaldehydes; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_527.pub2
