Titanium dioxide: a complete overview

What is Titanium Dioxide?
Titanium dioxide is an inorganic compound with the chemical formula TiO2. It’s a white solid that is insoluble in water. Titanium dioxide is found naturally as rutile, anatase, and brookite polymorphs.
Rutile and anatase are used industrially to produce pigments, catalysts, ceramics and various other technical materials.
Titanium dioxide is the most important white pigment because of its exceptional scattering properties, high chemical stability, and non-toxicity. In 2007, approximately 5.0 million metric tons of titaniumm dioxide pigments were produced.
Titanium dioxide is among the top five inorganic chemicals in terms of economic importance, with global annual sales exceeding $12 billion.
The production of titanium dioxide increased from 1.389 million tonnes in 1965 to 3.220 million tonnes in 1995. The sulfate process dominated early production (90.3% in 1965) but declined to 46.0% by 1995 as the chloride process expanded from 9.7% to 54.0%. Production capacity reached 5.915 million tonnes by 2008, with chloride process maintaining slight dominance at 53.2% versus sulfate process at 46.8%.
Table of Contents
1. Physical Properties of Titanium Dioxide
Titanium dioxide exists in three crystalline forms: rutile, anatase, and brookite. Rutile is the most thermodynamically stable. Despite this, anatase and brookite possess similar lattice energies, which contribute to their long-term stability. Anatase irreversibly converts to rutile above 700 °C. Brookite has limited industrial uses due to its production difficulty.
In all three titanium dioxide forms, each titanium atom is octahedrally coordinated by six oxygen atoms, and each oxygen atom is trigonally coordinated by three titanium atoms. The three crystalline structures correspond to different ways of linking the octahedra at their corners and edges.
Table 1 provides the crystal lattice constants and densities of these TiO2 modifications.
Rutile and anatase crystallize in the tetragonal system, while brookite crystallizes in the rhombic system. Titanium dioxide melts at approximately 1800 °C. Above 1000 °C, oxygen partial pressure increases due to oxygen liberation and the formation of lower titanium oxides. This process induces color changes and alters electrical conductivity. A reversible yellow coloration appears above 400 °C from thermal lattice expansion.
Rutile exhibits the highest density and atomic packing, resulting in superior hardness (Mohs 6.5–7.0) compared to anatase (Mohs 5.5–6.0). Additives can significantly influence the hardness of these materials in practical applications.
High refractive index combined with minimal visible spectrum absorption makes titanium dioxide an exceptional white pigment. Average refractive indices are 2.55 for anatase and 2.80 for rutile, which are wavelength-dependent.
Titanium dioxide is a light-sensitive semiconductor that absorbs near-UV electromagnetic radiation. The band gap energy that represents the energy difference between the valence and conduction bands is 3.03 eV for rutile and 3.15 eV for anatase, which correspond to absorption edges at 415 nm and 385 nm, respectively.
Based on the inflection point of the absorption curve, these values are 3.13 eV (397 nm) for rutile and 3.29 eV (377 nm) for anatase. Trace impurities, such as parts per million of iron, can cause visible light absorption and lead to a deviation from pure white.
Absorption of light energy excites an electron from the valence band to the conduction band. The resulting mobile electron and electron hole can migrate on the solid’s surface, where they can participate in redox reactions.
| Phase | CAS registry no. | Crystal system | Lattice constants, nm | Density, g/cm3 |
|---|---|---|---|---|
| Rutile | [1317-80-2] | tetragonal | a = 0.4594, c = 0.2958 | 4.21 |
| Anatase | [1317-70-0] | tetragonal | a = 0.3785, c = 0.9514 | 4.06 |
| Brookite | [12188-41-9] | rhombic | a = 0.9184, b = 0.5447, c = 0.5145 | 4.13 |
2. Chemical Properties of Titanium Dioxide
Titanium dioxide exhibits amphoteric properties with weak acidic and basic characteristics. Therefore, alkali-metal titanates and free titanic acids are unstable in aqueous environments and undergo hydrolysis to form amorphous titanium oxide hydroxides.
Titanium dioxide is chemically very stable and doesn’t react with most organic and inorganic reagents. Dissolution occurs in concentrated sulfuric acid upon prolonged heating and in hydrofluoric acid. Molten alkaline and acidic substances also attack and dissolve it.
At elevated temperatures, TiO2 reacts with reducing agents such as carbon monoxide, hydrogen, and ammonia to produce lower-valence titanium oxides without metallic titanium formation. Above 500 °C, titanium dioxide reacts with chlorine in the presence of carbon to generate titanium tetrachloride.
3. Surface Properties of Titanium Dioxide Pigments
Commercial titanium dioxide specific surface areas range from 0.5 to >300 m²/g depending on applications. Standard pigments exhibit 5-30 m²/g surface areas.
A titanium dioxide surface is naturally covered with coordinatively bonded water to form hydroxyl groups, making the surface of uncoated titanium dioxide polar. Hydroxyl surface coverage influences pigment characteristics such as dispersibility and weather resistance.
The presence of these surface hydroxyl groups makes various photochemical reactions possible, such as the photocatalytic decomposition of water into hydrogen and oxygen and the reduction of nitrogen to ammonia and hydrazine.
4. Industrial Production of Titanium Dioxide
Commercial titanium dioxide is primarily produced using two distinct industrial methods: the sulfate process and the chloride process. Both methods are designed to form titanium dioxide pigments.
The sulfate process is an older technique that begins by reacting titanium-rich raw materials, such as ilmenite, with concentrated sulfuric acid at high temperatures (150–220 °C). This reaction creates a solution containing titanium, iron, and other impurities. Through subsequent steps involving hydrolysis and purification, a titanium oxide hydrate is precipitated. This hydrate is then calcined (heated to high temperatures), ground, and often coated to yield the final pigment.
The chloride process involves chlorinating titanium-containing raw materials like ilmenite or rutile at even higher temperatures (700–1200 °C). This produces titanium tetrachloride (TiCl4), which is then oxidized at 900–1400 °C to form titanium dioxide. Similar to the sulfate process, the resulting TiO2 is then ground and coated.
It’s worth noting that while these two processes dominate pigment production, other specialized methods exist for producing titanium dioxide without pigment properties, particularly for nanoparticle synthesis. These alternative methods include techniques like the hydrolysis of titanium alcoholates or the pyrolytic reaction of titanium tetrachloride with water.
For detailed information on the production processes of titanium dioxide, visit the following article.
4.1. Production of Titanium Dioxide by the Sulfate Process
The sulfate process for titanium dioxide production involves a series of stages beginning with the grinding of titanium-containing raw materials to a fine powder (under 40 μm) after drying. This is followed by digestion, where the ground material is mixed with concentrated sulfuric acid (80–98%) at high temperatures (up to 220 °C), resulting in a solid digestion cake.
This cake is then dissolved and reduced in cold water or dilute acid to form a titanium dioxide solution, with trivalent iron being reduced to divalent iron to prevent contamination. Next, the solution undergoes clarification to remove all undissolved solids through settling and filtration. For ilmenite-derived solutions, iron sulfate is crystallized by cooling to reduce its concentration.
The key step is hydrolysis, where titanium oxide hydrate is precipitated from the solution at 94–110 °C, often using nuclei to control particle properties. This hydrolysate is then purified by reduction (bleaching) with agents like zinc or aluminum powder to remove remaining colored impurities, followed by a second filtration and washing.
Before the final heating step, the hydrate is doped with alkali-metal compounds and phosphoric acid, and sometimes rutile nuclei and stabilizers, to achieve specific pigment characteristics.
Finally, the doped hydrate is calcinated in rotary kilns at 800–1100 °C to remove water and residual sulfuric acid and convert the hydrate into crystalline titanium dioxide. The resulting clinker is then ground (wet or dry) to achieve the desired pigment fineness.
4.2. Production of Titanium Dioxide by the Chloride Process
The chloride process for producing titanium dioxide pigment begins with chlorination, where titanium-containing raw materials and petroleum coke are reacted with chlorine and oxygen in a fluidized-bed reactor at high temperatures (800–1200 °C) to produce titanium tetrachloride (TiCl4).
The resulting hot reaction gases are cooled, first to separate other solid chlorides and dust from the TiCl4, and then to condense most of the titanium tetrachloride by cooling it below 0 °C.
The condensed TiCl4 is then purified by distillation to remove solid chlorides and dissolved impurities like chlorine, vanadium tetrachloride, and phosgene to ensure a high-purity titanium tetrachloride product.
Finally, the purified TiCl4 vapor is heated and reacted with preheated oxygen at very high temperatures (900–1400 °C) in a specially designed reactor. This reaction produces titanium dioxide pigment and chlorine gas.
The TiO2 pigment is then separated from the gas stream, and the chlorine gas is often recycled back to the chlorination step. The quality of the pigment (particle size and distribution) is carefully controlled by factors such as reaction temperature and the addition of specific compounds.
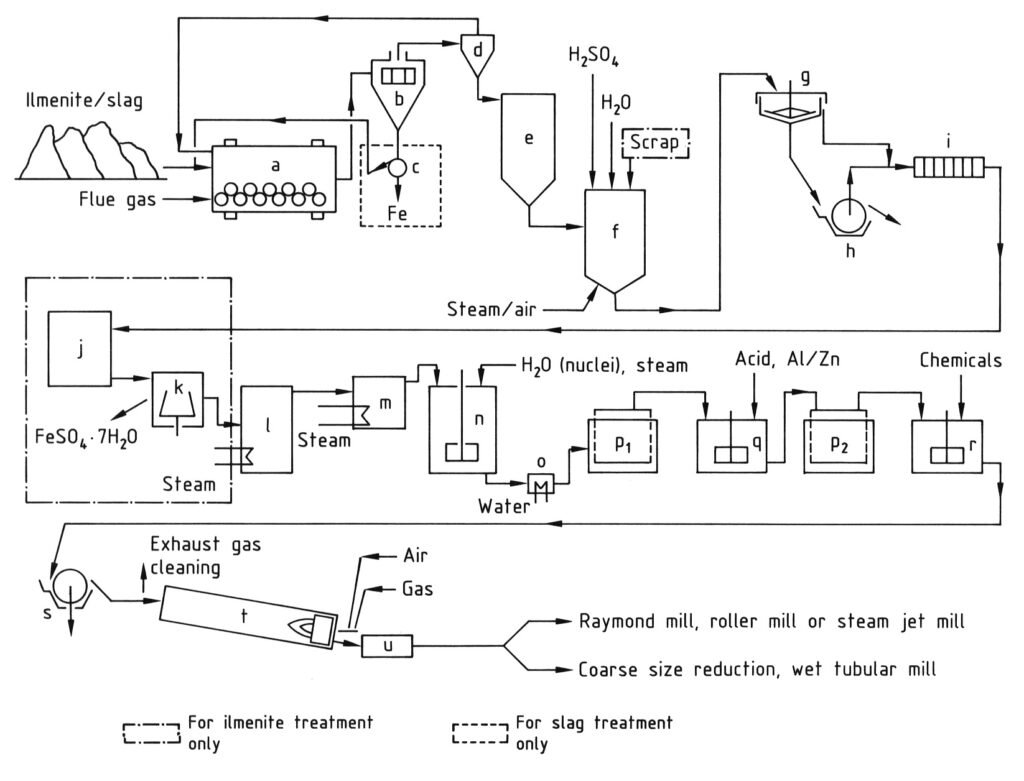
a) Ball mill/dryer; b) Screen; c) Magnetic separator; d) Cyclone; e) Silo; f) Digestion vessel; g) Thickener; h) Rotary filter; i) Filter press; j) Crystallizer; k) Centrifuge; l) Vacuum evaporator; m) Preheater; n) Stirred tank for hydrolysis; o) Cooler; p) Moore filters; q) Stirred tank for bleaching; r) Stirred tank for doping; s) Rotary filter for dewatering; t) Rotary kiln; u) Cooler
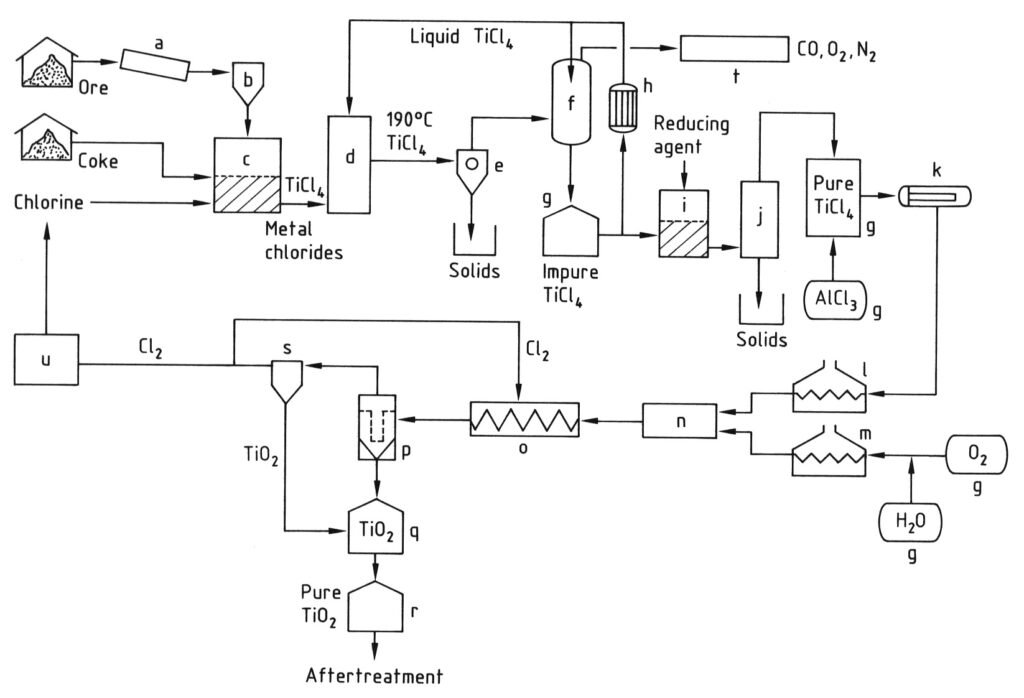
a) Mill; b) Silo; c) Fluidized-bed reactor; d) Cooling tower; e) Separation of metal chlorides; f) TiCl4 condensation; g) Tank; h) Cooler; i) Vanadium reduction; j) Distillation; k) Evaporator; l) TiCl4 superheater; m) O2 superheater; n) Burner; o) Cooling coil; p) Filter; q) TiO2 purification; r) Silo; s) Gas purification; t) Waste-gas cleaning; u) Cl2 liquefaction
5. Pigment Properties of Titanium Dioxide
The important pigment characteristics of titanium dioxide include scattering power, hiding power, brightness, masstone, gloss formation, gloss haze, dispersibility, lightfastness, and weather resistance. These properties depend on chemical purity, lattice stabilization, particle size distribution, and surface coatings. Property evaluation requires consideration of pigment-matrix interactions.
5.1. Scattering power
The refractive indices of rutile (n = 2.80) and anatase (n = 2.55) are even higher than diamond (n = 2.42). Relative refraction coefficients typically range from 1.5 to 2.0 across various binder systems.
Optimal scattering occurs at 0.2 μm particle size according to Mie theory. The scattering power depends on the wavelength; smaller particles of titanium dioxide scatter blue undertones, and larger particles scatter yellow undertones.
5.2. Masstone and color
Titanium dioxide whiteness depends on purity, crystalline modification, and particle size. Anatase pigments have less yellow undertone compared to rutile because their absorption band (385 nm) is shifted into the UV region.
The presence of transition element impurities affects whiteness, which is why the chloride process pigments have superior color purity because the TiCl4 is purified by distillation before oxidation.
5.3. Dispersion
Effective disintegration and dispersion of titanium dioxide pigments are essential for achieving high gloss and minimizing gloss haze. They are achieved by intensive grinding and organic surface coating. Surface treatment selection depends on application requirements.
5.4. Lightfastness and weather resistance
The weathering of paints and coatings containing titanium dioxide leads to pigment chalking. Absence of oxygen or low oxygen permeability in binders (e.g., melamine–formaldehyde resins) prevents chalking but causes reversible graying. Graying is significantly reduced in the absence of water.
Anatase shows greater susceptibility to both effects. Pigment manufacturers use stabilization methods, such as doping with zinc or aluminum before calcination or during oxidation.
In the presence of a stable binder, titanium dioxide pigments act as photocatalysts, inducing binder degradation, and with low-stability binders, they protect the binder from degradation. Consequently, durability testing requires highly stable binder systems.
The degradation mechanism follows a five-step cycle:
- Water adsorption forms surface hydroxyl groups.
- Short-wavelength light absorption (anatase <385 nm, rutile <415 nm) generates an electron and an electron hole (exciton) within the crystal lattice, which migrate to the pigment surface.
- At the surface, an OH– ion is oxidized to an OH• radical by the electron hole. This OH• radical desorbs and can oxidatively degrade the binder. Simultaneously, Ti4+ is reduced to Ti3+ by the remaining electron.
- The Ti3+ ion is oxidized by adsorbed oxygen to form an O2– ion, which reacts with H+ to yield an HO2• radical.
- The cycle ends with the binding of water to the regenerated TiO2 surface.
In summary, the chalking process is the reaction of water and oxygen to form OH• and HO2• radicals under the influence of shortwave radiation and the catalytic activity of the titanium dioxide surface.
This cycle can be interrupted by air or water exclusion. Oxygen exclusion or rate-limiting oxygen diffusion in the binder leads to Ti3+ ion accumulation and graying, which reverses upon gradual oxygen exposure. Water exclusion prevents rehydration and surface hydroxyl formation.
Despite this photochemical activity, treated rutile pigments stabilize many binders by preventing light penetration into coating layers. High-quality TiO2 pigments must demonstrate excellent weather resistance, such as resisting significant chalking or gloss deterioration after a two-year Florida test exposure.
5.5. Abrasivity
Abrasivity is an undesirable property of titanium dioxide pigments. Anatase has lower abrasivity (Mohs 5.5-6.0) compared to rutile (Mohs 6.5-7.0). While these intrinsic hardness values appear similar, doping additives significantly influence practical hardness.
For example, alumina-doped rutile pigments have considerably higher abrasivity than anatase pigments without alumina. Pigments from the chloride process, typically containing more alumina, often show higher abrasivity than sulfate pigments.
This phenomenon is attributed to the segregation of acceptor ions like Al2O3 to the surface during calcination even at low concentrations (< 0.4%). Therefore, studies on titanium dioxide surface topology and lattice parameters require ultrahigh-purity materials to prevent misinterpretation.
6. Uses of Pigmentary Titanium Dioxide
Titanium dioxide has achieved universal adoption, nearly replacing other white pigments. In 2007, Asia leads global consumption, followed by Europe and North America. Rutile comprises approximately 90% of total consumption, and anatase accounts for 10%.
6.1. Uses in paints and coatings
Paints and coatings are the largest consumer of titanium dioxide. The TiO2 pigment enhances the protective capabilities of coating materials. Modern TiO2 pigments can form thin coatings (a few micrometers) to provide complete substrate coverage. Pigment volume concentrations range from 10% to 35% in glossy paints and can exceed 80% in matte emulsion paints.
Commercial pigments allow paint manufacturing using basic dispersion equipment such as disk dissolvers. Organic treatment before steam-jet micronization improves gloss properties and reduces gloss haze in stoving enamels. Sedimentation does not occur when these products are stored, and they possess good lightfastness and weather resistance.
6.2. Uses in printing inks
Modern printing operates at coating thicknesses below 10 μm, requiring the finest possible titanium dioxide pigments. These ultra-thin films are only possible with TiO2 pigments that have a lightening (reducing) power seven times that of lithopone.
Exceptional dispersibility proves essential for gloss achievement. Titanium dioxide’s neutral masstone makes it particularly suitable for lightening colored pigments.
6.3. Uses in plastics
Titanium dioxide is extensively used to color both durable and non-durable plastic goods, including toys, appliances, automobiles, furniture, and packaging films. TiO2 pigments absorb UV radiation below 415 nm; therefore, they are used to protect pigmented plastics from harmful rays.
Even minimal titanium dioxide imparts opacity to plastics, so it is added to dark-colored products to prevent a translucent, “sleazy” appearance.
6.4. Uses in fibers
Titanium dioxide pigments provide a solid appearance to synthetic fiber by eliminating translucent properties and the associated greasy appearance. Anatase pigments are preferred in this application due to their lower abrasive effect on spinning equipment compared to rutile. Appropriate coating can increase the poor lightfastness of anatase pigments in polyamide fibers.
6.5. Uses in paper
In Europe, fillers such as kaolin, chalk, or talc are commonly used for paper brightening and opacifying. Titanium dioxide pigments are ideal for high-whiteness paper requiring opacity at very thin gauges (e.g., airmail or thin printing paper).
TiO2 can be added into the paper pulp or applied as a coating for superior “art” paper quality. Laminated papers typically are colored with extremely lightfast rutile pigments before impregnation with melamine–urea resin for decorative layers or films.
6.6. Additional applications
Titanium dioxide pigments are used in the enamel and ceramic industries, white cement manufacturing, and the coloring of rubber and linoleum. Additionally, they are used as UV absorbers in sunscreens, soaps, cosmetic powders, creams, toothpaste, and cigar wrappers.
Titanium dioxide is also used as a food colorant and in the broader cosmetics industry due to its properties such as non-toxicity (oral intake), compatibility with skin and mucous membranes, and good dispersibility in various solutions and binders.
Electrically conducting TiO2 pigments have been produced by surface treatments with indium-tin or antimony-tin mixed oxide coatings. These pigments are used in fibers for electrophotographic papers and in the production of antistatic plastics.
7. Uses of Nonpigmentary Titanium Dioxide
Non-pigmentary TiO2 is used in vitreous enamels, glass and glass ceramics, electroceramics, catalysts and catalyst supports, welding fluxes, colored pigments, electrical conductors, chemical intermediates (e.g., potassium fluorotitanate), structural ceramics, UV absorbers, and refractory linings.
7.1. Electroceramics
Titanates (e.g., barium, strontium, calcium, and lead titanate), synthesized from high-purity titanium dioxide substrates, are used in the production of capacitors, PTC resistors, and piezoelectric materials.
Requirements for TiO2 starting materials regarding purity, reactivity, and sintering characteristics are becoming more stringent due to the miniaturization trend in electronic components, necessitating thinner ceramic layers and smaller particles.
Recent advancements using titanium oxide hydrate (from the sulfate process) have allowed the solid-state production of ultrapure, ultrafine BaTiO3 below 500 °C.
Piezoceramic multilayer components, such as actuators for fuel-injection systems, require low-sintering ceramics. Highly reactive TiO2 and ZrO2 components for lead zirconate titanate (PZT) facilitate perovskite structure formation and subsequent sintering at temperatures low enough to significantly reduce the expensive palladium content in the electrodes of these actuators.
Global titanium dioxide consumption for electroceramics exceeds 10,000 tons/year. Despite the effect of miniaturization of electroceramic components resulting in less raw material required per electronic piece, continued annual growth can be anticipated.
7.2. Catalysts
Titania is used as an active catalyst for diverse thermal and photochemical reactions, involving both inorganic and organic compounds. It can be self-supported or deposited on other materials. Doping with additional elements typically enhances its desired catalytic effect.
The primary catalytic application of titanium dioxide is in the removal of nitrogen oxide from industrial waste gases (power stations, incinerators and diesel automotives). In this process called selective catalytic reduction (SCR), nitrogen oxides react with ammonia in the presence of oxygen over the catalyst to produce nitrogen and water.
The global consumption of titanium dioxide for SCR catalysts is approximately 25,000 t/a. These catalysts typically contain up to 15% tungsten oxide and about 1% V2O5 alongside TiO2. Catalyst manufacturing methods include extrusion into honeycomb shapes, coating onto substrates (e.g., cordierite), or layering onto metal sheets.
Strict requirements exist for purity, particle size, and porosity to ensure sustained catalytic activity over long lifetimes. Increasing demand for nitrogen oxide removal from stationary and mobile diesel engines is expected.
Numerous other catalytic applications utilize pure TiO2, TiO2 with incorporated additives, or TiO2 with surface-deposited metals (e.g., Rh, Pt, Pd, Au, Ag).
7.3. Photocatalysts
Extensive global research focuses on harnessing the photoactivity of untreated or treated titanium dioxide nanoparticles. Titanium dioxide can be used for the catalytic decomposition of organic compounds in wastewater or for photocatalytic air purification.
Pure TiO2 photocatalysis requires UV radiation due to its semiconductor bandgap of approximately 3.1 eV, so it is only used for outdoor applications. However, doping of titanium dioxide with metals, carbon, or nitrogen enables photocatalytic reactions using the visible part of the spectrum. Nevertheless, radiation intensity can be a limiting factor even for visible light applications.
Examples of recent photocatalytic applications are self-cleaning architectural (outdoor) coatings, interior coatings for air pollutant decomposition in buildings, and floor tiles, coatings, or concrete incorporating nanostructured titanium dioxide for urban nitrogen oxide decomposition.
These technologies are limited to specialized applications, such as hospitals or locations of specific public interest.
7.4. Mixed metal oxide pigments
Anatase pigments, or titanium dioxide hydrolysates, are calcined with transition metal oxides to form chromium rutile or nickel rutile pigments (mixed metal oxide pigments).
7.5. UV absorption
Nanostructured TiO2 particles (5–50 nm) are incorporated into sunscreens for the cosmetic industry and for UV protective coatings (e.g., for wood). Usually, nanostructured rutile particles with inorganic and optional organic coatings are used.
Nanosized titanium dioxide appears transparent; it effectively absorbs UV-B (280–320 nm) and UV-A (320–400 nm) radiation. Due to their small particle size, these nanostructured titanium dioxide particles require more inorganic or organic coating compared to pigmentary TiO2.
7.6. Other applications
Non-pigmentary titanium dioxide is also used in batteries, fuel cells, water cleavage, and photovoltaic systems. Lithium titanate-based batteries are gaining interest as rechargeable energy storage systems for various applications.
While the TiO2-based photovoltaic systems (Grätzel cells) are well-understood, the application of titanium dioxide in fuel cells and for photocatalytic hydrogen generation from water using sunlight still requires substantial research.
8. Toxicology of Titanium Dioxide
Titanium dioxide exhibits good stability and complete nontoxicity upon oral administration. Long-term animal studies with dietary TiO2 show no evidence of titanium uptake.
Historically, the absorption of finely divided titanium dioxide pigments in the lungs was not associated with specific carcinogenic effects. Consequently, the International Agency for Research on Cancer (IARC) initially classified titanium dioxide as a Class 3 substance, meaning it was “not classifiable as to its carcinogenicity to humans.”
However, in 2007, the IARC reclassified TiO2 as a Class 2B substance, indicating it is “possibly carcinogenic to humans.” This classification applies to both pigment-grade titanium dioxide (primary particle size 200–300 nm) and ultrafine grades (primary particle size less than 50 nm).
Despite this reclassification, epidemiological studies on titanium dioxide dust exposure in workers have not shown increased mortality or incidence of lung cancer.
References
- Auer, G., Woditsch, P., Westerhaus, A., Kischkewitz, J., Griebler, W.-d., Rohe, M. and Liedekerke, M. (2017). Pigments, Inorganic, 2. White Pigments. In Ullmann’s Encyclopedia of Industrial Chemistry, (Ed.). https://doi.org/10.1002/14356007.n20_n01.pub2
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. “Titanium Dioxide: From Engineering to Applications.” Catalysts, 2019, 9 (2), 191. DOI: 10.3390/catal9020191.
- Haider, A. J.; Jameel, Z. N.; Al-Hussaini, I. H. M. “Review on: Titanium Dioxide Applications.” Energy Procedia, 2019, 157, 17-29. DOI: 10.1016/j.egypro.2018.11.159.
- Fujishima, A.; Rao, T. N.; Tryk, D. A. “Titanium dioxide photocatalysis.” Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 2000, 1 (1), 1-21. DOI: 10.1016/S1389-5567(00)00002-2.
- Diebold, U. “The surface science of titanium dioxide.” Surface Science Reports, 2003, 48 (5–8), 53-229. DOI: 10.1016/S0167-5729(02)00100-0.
- Chen, X.; Mao, S. S. “Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications.” Chem. Rev., 2007, 107 (7), 2891–2959. DOI: 10.1021/cr0500535.
- Racovita, A. D. “Titanium Dioxide: Structure, Impact, and Toxicity.” Int. J. Environ. Res. Public Health, 2022, 19 (9), 5681. DOI: 10.3390/ijerph19095681.
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. “Titanium Dioxide Nanoparticles in Food and Personal Care Products.” Environ. Sci. Technol., 2012, 46 (4), 2242–2250. DOI: 10.1021/es204168d.
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. “Investigating the use of titanium dioxide (TiO2) nanoparticles on the amount of protection against UV irradiation.” Sci Rep, 2023, 13, 9793. DOI: 10.1038/s41598-023-37057-5.
- Middlemas, S.; Fang, Z. Z.; Fan, P. “A new method for production of titanium dioxide pigment.” Hydrometallurgy, 2013, 131–132, 107-113. DOI: 10.1016/j.hydromet.2012.11.002.
- Gázquez, M.; Bolívar, J.; Garcia-Tenorio, R.; Vaca, F. “A Review of the Production Cycle of Titanium Dioxide Pigment.” Materials Sciences and Applications, 2014, 5, 441-458. DOI: 10.4236/msa.2014.57048.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. “Titanium Dioxide.” In Some Organic Solvents, Resin Monomers and Related Compounds, Pigments and Occupational Exposures in Paint Manufacture and Painting; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 47; International Agency for Research on Cancer: Lyon (FR), 1989. Available from: https://www.ncbi.nlm.nih.gov/books/NBK524874/.
- Goparaju, V. R. R.; Marshall, D. F.; Kazerooni, V. “Process for manufacturing titanium dioxide pigments using ultrasonication.” U.S. Patent 9,353,266, 2016. Available from: https://patents.google.com/patent/US9353266B2/en.
- Davis, B. R.; Rahm, J. A. “Process for manufacturing titanium dioxide.” U.S. Patent 4,288,418, 1981. Available from: https://patents.google.com/patent/US4288418A/en.
- Ayorinde, T.; Sayes, C. M. “An updated review of industrially relevant titanium dioxide and its environmental health effects.” J. Hazard. Mater. Lett., 2023, 4, 100085. DOI: 10.1016/j.hazl.2023.100085.
