Thioglycolic Acid: Properties, Reactions, Production and Uses
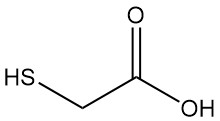
What is thioglycolic acid?
Thioglycolic acid, also known as mercaptoacetic acid, is the simplest and industrially most important mercaptocarboxylic acid with the formula HSCH2COOH. It is a colorless liquid with a strong, unpleasant odor.
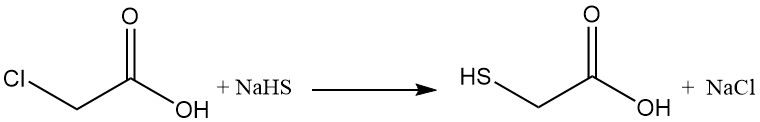
Mercaptoacetic acid was first synthesized in 1862 by Carius by reacting chloroacetic acid with potassium hydrogen sulfide.
Table of Contents
1. Physical Properties of Thioglycolic Acid
Thioglycolic acid is a clear, colorless liquid with a characteristic odor. It is miscible with water, mono- and polyalcohols, ethers, ketones, esters, chlorinated hydrocarbons, and aromatic hydrocarbons, but insoluble in aliphatic hydrocarbons.
The esters of thioglycolic acid are mostly colorless liquids with characteristic odors. The cetyl and stearyl esters are pale yellow waxes.
Some physical properties of mercaptoacetic acid are summarized in the following table.
| Property | Value |
|---|---|
| CAS number | [68-11-1] |
| Formula | HSCH2COOH |
| Molecular mass | 92.11 g/mol |
| Melting point | -16.5 °C |
| Boiling point at 2.3 kPa | 110–112 °C |
| Boiling point at 0.1 kPa | 79 – 80 °C |
| Refractive index | 1.5027 |
| Density | 1.325 g/cm3 |
| pKa1 | 3.82 |
| pKa2 | 9.30 |
| Heat of combustion | 1450 kJ/mol |
| Vapor pressure (30 °C) | 0.02 kPa |
| Vapor density | 3.18 |
| Flash point | 126 °C |
2. Reactions of Mercaptoacetic acid
Mercaptoacetic acid (thioglycolic acid) possesses two reactive functional groups: a thiol (mercapto) group and a carboxylic acid group. This bifunctionality allows it to participate in various reactions, forming diverse products.
Thioglycolic acid readily forms salts with both its carboxylate and thiol groups. These include metal salts, esters, amides, anilides, and thioethers.
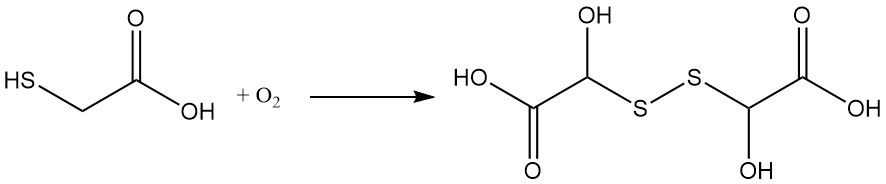
Mercaptoacetic acid undergoes oxidation, even upon exposure to air. This reaction leads to the formation of dithiodiglycolic acid. Trace metals like copper, iron, and manganese can catalyze this oxidation.
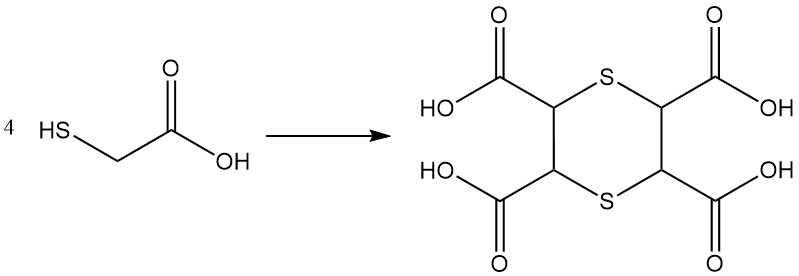
Concentrated solutions (80%) of thioglycolic acid undergo condensation reactions to yield linear and cyclic polycondensation products; examples include the formation of a tetracarboxylic acid with a 1,4-dithiane ring structure.
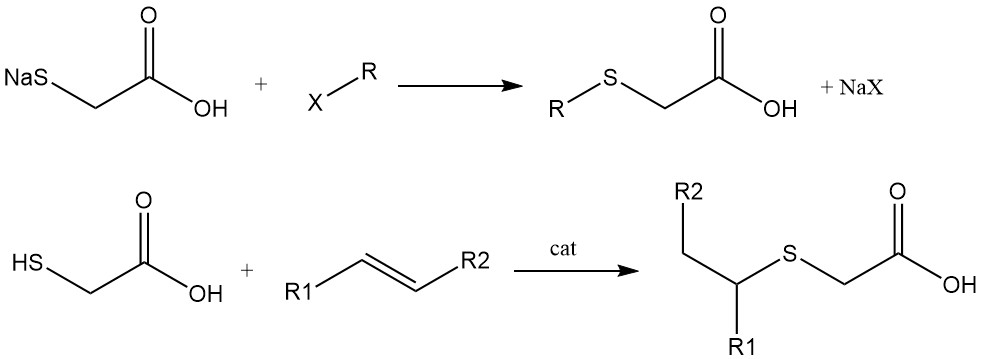
Thioethers are prepared by reactions between the sodium or potassium salts of the mercapto group and alkyl halides, or by the addition of mercaptoacetic acid or its derivatives to double or triple bonds. The regioselectivity of these reactions (Markovnikov vs. anti-Markovnikov) depends on the chosen catalyst.
Thioglycolic acid and its derivatives react with aldehydes and ketones to form various products, including mercaptals, mercaptols, or α,β-unsaturated thioethers. These reactions are catalyzed by acids, such as mineral acids or toluenesulfonic acids.
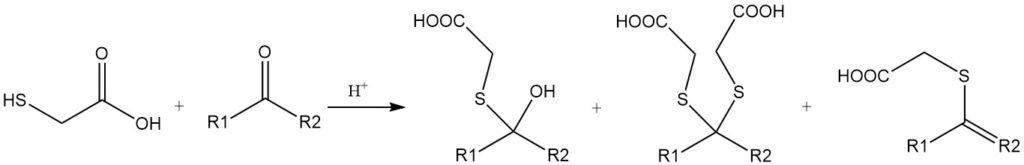
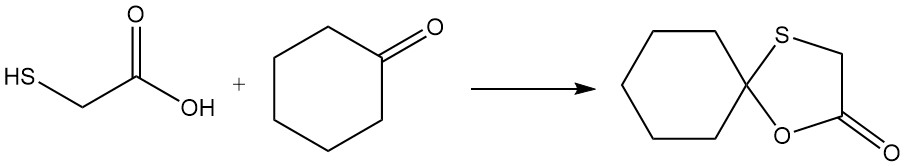
Mercaptoacetic acid can participate in a unique reaction where both the mercapto and carboxyl groups react simultaneously with a carbonyl group. An example is the reaction with cyclohexanone, which yields 2,2-pentamethylene-1,3-oxathiolane-5-one.
The strong reducing nature of thioglycolic acid is used to study biochemical redox systems and enzyme activity. Additionally, its reducing properties are employed in various industrial processes like cold-waving hair preparations and wool modification.
The use of mercaptoacetic acid in permanent hair waving is based on its ability to reduce and cleave the disulfide bridges in hair keratin.
3. Production of Thioglycolic Acid
Thioglycolic acid (mercaptoacetic acid) is commercially produced by the reaction of chloroacetic acid or its salts with alkali metal hydrogen sulfides (NaHS or KHS). This reaction produces undesired byproducts such as thiodiglycolic acid, dithiodiglycolic acid, and glycolic acid.
The isolation of the desired product is achieved by acidifying the reaction mixture, followed by extraction with organic solvents like ethers, alcohols, or chlorinated hydrocarbons, and then the crude product is purified by distillation.
Production methods use both batch and continuous processes, with some variations employing a partial pressure of hydrogen sulfide or carbon dioxide.
Alternative processes utilize waste streams from aromatic nitro compound reduction as a source of sulfur instead of alkali metal hydrogen sulfides. Also, a specific process is based on the cleavage of imidazolyl mercaptoacetic acid with sodium sulfide.
Several other routes exist for mercaptoacetic acid synthesis:
- Cleavage of the Bunte salt (NaO3SSCH2COOH) derived from chloroacetic acid and sodium thiosulfate using dilute sulfuric acid.
- Reduction of dithioglycolic acid prepared from chloroacetic acid and alkali metal polysulfides.
- Decomposition of the xanthate ester formed from chloroacetic acid and potassium ethylxanthate.
Anhydrous mercaptoacetic acid is produced by distillation of the technical-grade product with toluene. Contact with metal catalysts during production must be minimized to prevent dimerization of the anhydrous thioglycolic acid.
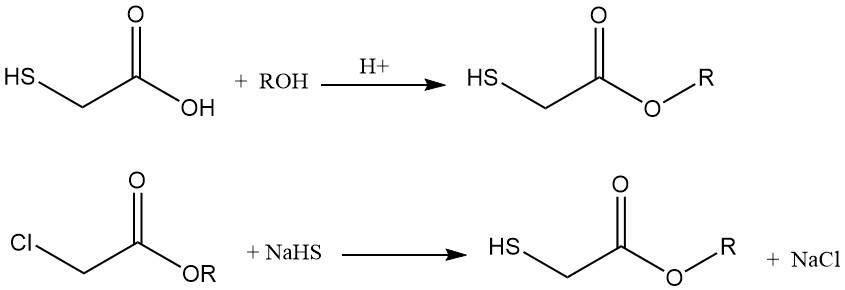
Esters of thioglycolic acid can be synthesized via two main methods:
- Esterification with alcohols, where the alcohol can be used as the extraction solvent after acid synthesis.
- Reaction of the corresponding alkyl chloroacetate with sodium thiosulfate.
4. Uses of Thioglycolic Acid
Mercaptoacetic acid (thioglycolic acid) and its derivatives have found diverse applications across various industries since around 1950.
Ammonium, ethanolammonium salts, and increasingly, glycerol monothioglycolate are used to formulate cold-waving solutions for hair styling. The calcium salt of thioglycolic acid is used in depilatory creams for hair removal.
Mercaptoacetic acid derivatives are employed in permanent waving processes for wool, particularly in hat-making.
Organotin compounds derived from thioglycolic acid, like dialkyltin bis (isooctyl thioglycolate) and alkyltin tris (isooctyl thioglycolate), are highly effective thermal stabilizers for polyvinyl chloride (PVC). Their low toxicity and minimal migration make them suitable for food-grade packaging materials and drinking water pipes.
Esters, especially those derived from higher alcohols like isooctanol and decanol and amides of mercaptoacetic acid, are used as catalysts and antioxidants during the production of various plastics and rubbers.
The sodium salt of thioglycolic acid is a component of Brewer’s nutritive medium used to cultivate anaerobic bacteria.
Mercaptoacetic acid is used in analytical chemistry to separate iron and aluminum and as a reagent for detecting various metals, including iron, nitrite, uranium, vanadium, chromium, copper, molybdenum, and palladium.
It is also used as a matrix compound in fast atom bombardment mass spectrometry, a technique for analyzing biomolecules, and in the mild isolation process of lignin in combination with boron trifluoride.
In the pharmaceutical industry, thioglycolic acid derivatives are used as starting materials for the synthesis of thiophene and dihydrothiophene. S-aryl mercaptoacetic acids are intermediates used in the production of dyes.
Global production of thioglycolic acid is estimated to be in the range of 15,000 to 20,000 tons per year.
5. Toxicology of Thioglycolic Acid
Direct contact with thioglycolic acid can irritate the skin and eyes. Inhalation of mercaptoacetic acid vapors can irritate the mucous membranes, leading to coughing and shortness of breath, delayed wound healing, and blisters.
Due to these potential health risks, it is crucial to wear protective gloves and eyewear when handling thioglycolic acid.
The threshold limit value (TLV) established for mercaptoacetic acid is 1 ppm (5 mg/m³), which signifies the maximum airborne concentration for an 8-hour workday and a 40-hour workweek to which most workers can be repeatedly exposed without adverse health effects.
Studies indicate that the oral LD50 (lethal dose for 50% of the test population) in rats for thioglycolic acid is 261 mg/kg.
Generally, derivatives of mercaptoacetic acid and commercially available products containing them are significantly less toxic than pure acid. Additionally, legal regulations often dictate safe maximum concentrations for these products, minimizing health risks during their intended use.
Reference
- Mercaptoacetic Acid and Derivatives; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a16_265
