Sodium glutamate: production, properties and uses
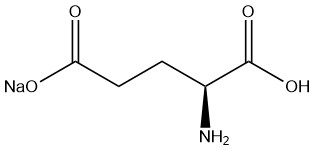
Monosodium glutamate (MSG) is a sodium salt of L-glutamic acid, which is widely used as a flavor enhancer in the food industry. According to Nikkan Keizai Tsushinsha Co., Ltd., MSG production reached 3 million tonnes in March 2013.
L-Glutamic acid is one of the most abundant amino acids naturally occurring in proteins or as a free amino acid in solution. It is widely used in commercial foods such as packaged and prepared foods, ready-made soups, and as a table seasoning.
In 1866, RITTHAUSEN isolated L-glutamic acid from the acid hydrolysate of wheat gluten. Later in 1890, WOLFF identified the chemical structure of L-glutamic acid through synthetic means.
KIKUNAE IKEDA discovered the flavor-enhancing properties of the monosodium salt of glutamic acid in 1908.
Table of Contents
1. Production of sodium glutamate
1.1. Classical Processes
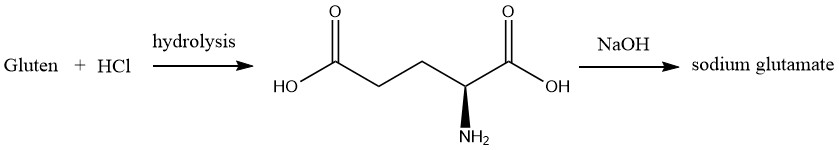
Industrial production of monosodium glutamate (MSG) commenced in 1909 and continued until 1965. During this time, the main raw material used was wheat gluten that was separated from wheat flour due to its high content of L-glutamic acid (approximately 25 wt%).
After 1934, defatted soybean flake was also utilized as a raw material as it did not produce starch as a byproduct.
The hydrolysis of raw materials involved heating with hydrochloric acid. Following concentration under reduced pressure and the addition of concentrated hydrochloric acid, L-glutamic acid hydrochloride crystals were formed by cooling the hydrolysate.
Due to its low solubility in concentrated hydrochloric acid, L-glutamic acid can be easily separated from other amino acids in the form of its hydrochloride.
The L-glutamic acid hydrochloride crystals were collected by filtration, dissolved in warm water, and the solution was filtered to remove any insoluble humic materials formed by the reaction of amino acids with carbohydrates.
The pH of the filtrate was adjusted to 3.2, the isoelectric point of L-glutamic acid, with sodium hydroxide or ammonia to precipitate L-glutamic acid crystals.
The yield of L-glutamic acid was high due to its low solubility in water (0.864 g per 100 mL at 25°C). The crude L-glutamic acid crystals were suspended in water and neutralized with sodium hydroxide.
The solution was then decolorized with activated charcoal, resulting in a clear solution which was concentrated to crystallize MSG crystals. The crystals of MSG were separated from the mother liquor through centrifugation and dried for packaging.
1.2.Production of Sodium Glutamate by Fermentation

In the early 1950s, DAGLEY and colleagues reported the presence of small amounts of amino acids in the culture medium of Escherichia coli. They observed that the addition of excess ammonium salts resulted in an increase in the production of amino acids.
In 1957, KINOSHITA et al. discovered bacteria that accumulate significant amounts of L-glutamic acid. The KINOSHITA et al. method using Micrococcus glutamicus (later renamed Corynebacterium glutamicum) gave yields of L-glutamic acid of about 30%, which was immediately applied to industrial production.
Bacteria that produce L-glutamic acid are widely distributed in nature, and most of these bacteria are Gram-positive, non-spore-forming, nonmotile, and require biotin for growth. For industrial production of monosodium glutamate (MSG), molasses and starch hydrolysate are commonly used as raw materials. The accumulation of L-glutamic acid is governed not by its biosynthesis in the bacterial cells, but by its secretion.
Biotin is one of the essential vitamins for cell growth, but a biotin concentration in the culture medium that is sufficient for cell growth makes the cell membranes impermeable for L-glutamate, resulting in poor accumulation.
The critical biotin content of the cells for L-glutamic acid production is 0.5 mg/g dry cells. Thus, the accumulation of L-glutamic acid is at a maximum when the biotin concentration is suboptimal for maximum growth.
Therefore, raw materials that are rich in biotin, such as beet molasses and cane molasses, could not be used until the discovery of the biotin-inhibiting effects of C16–C18 saturated polyoxyethylene fatty acid esters.
Limiting the amount of biotin is believed to cause incomplete biosynthesis of oleic acid, which results in a decrease in the phospholipids in the cell membrane and makes the membrane permeable.
Ammonium salts and urea are used as nitrogen sources for both microbial growth and product formation. The assimilation of ammonium ions and formation of L-glutamic acid cause the culture medium to become acidic.
Gaseous ammonia is used advantageously to maintain neutral pH and avoid dilution of the broth because it contains neither hydroxyl ions nor water.
As the biosynthesis of L-glutamic acid is an aerobic process, the pressure of dissolved oxygen must strictly be kept above 1 kPa by aeration and agitation in the fermenter.
Progress in fermentation technology has made it possible to raise the accumulation and yield of L-glutamic acid above 120 g/L and 63%, respectively.
The pH of the fermentation broth is adjusted to 3.2 to recover L-glutamic acid crystals, which are then converted to MSG by the same method used in the extraction process.
2. Properties of sodium glutamate
L-Glutamic acid undergoes intramolecular peptide bond formation between its α-amino and γ-carboxyl groups upon dehydration. This reaction exhibits a preference for the formation of 2-pyrrolidone-5-carboxylic acid (PCA) in weakly acidic or weakly basic pH conditions.
At neutral pH, dehydration occurs at a lower rate. Conversely, strong acidic or alkaline conditions promote the hydration of PCA.
Cooking conditions that are normally encountered do not result in racemization or dehydration.
The minimum perceptible concentration of MSG is 0.03%, which is smaller than the threshold values of sodium chloride (0.2%) or sucrose (0.5%). The flavor enhancing activity of MSG is synergistically increased in the presence of 5′-inosinate and 5′-guanylate.
L-Glutamic acid is classified as a non-essential amino acid and plays a critical role in the nitrogen metabolism of amino acids. Glutamate dehydrogenase catalyzes the reductive amination of 2-oxoglutarate to L-glutamate for the incorporation of inorganic nitrogen.
Many organisms possess a variety of aminotransferases that transfer amino groups from amino acids to 2-oxo-acids, and many of these enzymes use the glutamate/2-oxoglutarate pair as the amino donor/acceptor.
L-Glutamate serves as a direct precursor for various essential substances, including L-glutamine, the neurotransmitter γ-aminobutyric acid (GABA), γ-carboxyglutamate in calcium binding proteins, and the tripeptide glutathione.
3. Uses of monosodium glutamate
Seasoning
As a well-established seasoning or flavor enhancer, MSG is widely used to improve the palatability of foods. The characteristic taste of MSG, umami, distinguishes it from the other four basic tastes: sweet, salty, sour, and bitter.
Recent advances in molecular biology have identified the proteins that function as umami taste receptors, which are expressed in specialized cells of taste buds on the tongue and through the oral cavity.
Glutamates are present in various foods such as human milk, tomatoes, cheese, seaweed, fish, meats, and vegetables. Other umami substances, such as 5′-ribonucleotides like inosinate and guanylate, are also found in meats, fish, and dried mushrooms.
Monosodium glutamate is effective in enhancing the flavor of a wide variety of foods, especially meats, seafood, vegetables, soups, stews, and chowders.
However, alcoholic beverages, fruits, and confectionery are not improved by the addition of MSG, and its effect on smell or aroma is negligible.
Industrial Uses
Glutamic acid is used for industrial applications, depending on the pH. For instance, arginine glutamate is used as a raw material for pharmaceuticals and cosmetics.
Glutamic acid hydrochloride, which is produced by reacting glutamic acid with hydrochloric acid, is used as a gastric acidifier.
Glutamic acid’s amphoteric electrolyte properties are exploited in chelating agents, buffers, and builders for detergents.
The sodium salt of pyroglutamic acid, obtained by neutralizing the dehydrated glutamic acid with sodium hydroxide, is very hygroscopic and is used as a component of natural moisturizing factors for human skin and as a humectant in cosmetics.
A polymer of methyl glutamate serves as a coating agent for synthetic leather.
A condensate with formaldehyde acts as a retarder for plaster.
The N-acylated compound produced by the reaction with a long-chain fatty acid is an anionic surfactant that is popularly used in skin and hair cleansers due to its mildness and high safety.
The dibutylamide of this N-acylglutamic acid gelatinizes nonpolar oils and can be used as a recovering agent for spilled marine oil.
Reference
Monosodium Glutamate; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a16_711.pub2
