Nitrobenzoic Acid: properties, reactions and production
Nitrobenzoic acids are derivatives of benzoic acid with the general formula C7H5NO4. Two isomers (meta and para) are commercially important. Nitrobenzoic acids are about ten times more acidic than benzoic acid because of the presence of the nitro groupe.
Table of Contents
2-Nitrobenzoic acid or o-nitrobenzoic acid

2-Nitrobenzoic acid is mono nitrobenzoic acid where the nitro group is on position ortho to the carboxylic group. It is a solide that is insoluble in water an soluble in alcohol like methanol.
Physical properties of 2-nitrobenzoic acid
The physical properties of the 2-nitrobenzoic acid are listed below:
- Molar mass = 167.12 g/mol
- Melting point = 148 °C
- Density = 1.575
- Solubility in water = 0.75 g/100 ml (at 25 °C)
- Solubility in methanol = 42.72 g/100 ml (at 10 °C)
- Solubility in benzene = 0.294 g/100 ml (at 10 °C)
- Dissociation constant, Ka (at 18 °C) = 6.1 × 10-3
- Decarboxylation temperature = 180 °C
Chemical reactions of 2-nitrobenzoic acid
2-nitrobenzoic acid undergoes the typical reaction of aromatic carboxylic acids and nitroaromatic compounds:
Anthranilic acid can be produced by the reduction of the nitro group into an amine.
The carboxylic group forms esters with alcohols and 2-nitrobenzoyl chloride with chlorinating agents.
At temperatures above 180 °C, 2-nitrobenzoic acid forms nitrobenze by decarboxylation.
The aromatic ring can undergo substitution reactions, but it is difficult due to the presence of deactivating groups.
Production of 2-Nitrobenzoic acid
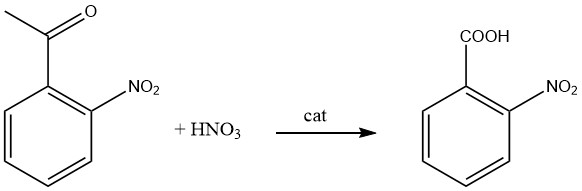
2-Nitrobenzoic acid is prepared by the oxidation of 2-nitrotoluene with nitric acid.

Monsanto Chemicals Ltd. had developed a production method for 2-nitrobenzoic acid by oxidation of 2-nitroacetphenone with aqueous nitric acid in the presence of ammonium metavanadate as a catalyst under refluxing.
Eight moles of nitric acid are required to convert one mole of 2-nitroacetophenone at a temperature of 60 °C to the refluxing temperature of the mixture.
By cooling the reaction mixture, the crude 2-nitrobenzoic acid crystals form and are recovered therefrom by filtration, then purified by recrystallization from such solvents as benzene, ethanol, benzene-ethanol mixtures, hexane, etc.
3-nitrobenzoic acid or m-nitrobenzoic acid

3-Nitrobenzoic acid is a white solid. The carboxylic and nitro substituents are in a meta position with respect to each other, which is why it is also called m-nitrobenzoic acid. It is an important precursor in the production of dyes.
Physical properties of 3-nitrobenzoic acid
The physical properties of the 3-nitrobenzoic acid are listed below:
- Molar mass = 167.12 g/mol
- Melting point = 142 °C
- Density = 1.494
- Solubility in water = 0.24 g/100 ml (at 15 °C)
- Solubility in methanol = 47.34 g/100 ml (at 10 °C)
- Solubility in benzene = 0.795 g/100 ml (at 10 °C)
- Dissociation constant, Ka (at 25 °C) = 3.48 × 10-4
- Decarboxylation temperature = 238 °C
Chemical reactions of 3-nitrobenzoic acid
3-nitrobenzoic acid undergoes the typical reaction of aromatic carboxylic acids and nitroaromatic compounds:
3-Aminobenzoic acid can be produced by the reduction of the nitro group into an amine.
The carboxylic group forms esters with alcohols and 3-nitrobenzoyl chloride with chlorinating agents.
At temperatures above 238 °C, 3-nitrobenzoic acid forms nitrobenze by decarboxylation.
The aromatic ring can undergo substitution reactions, but it is difficult due to the presence of deactivating groups.
Production of 3-nitrobenzoic acid
The 3-Nitrobenzoic Acid is prepared by the nitration of benzoic acid under low-temperature conditions. During this process, around 20% of the 2-nitro isomer and 1.5% of the 4-nitro isomer are produced alongside the desired 3-Nitrobenzoic Acid. To obtain purified 3-Nitrobenzoic Acid, recrystallization of the sodium salt can be employed.
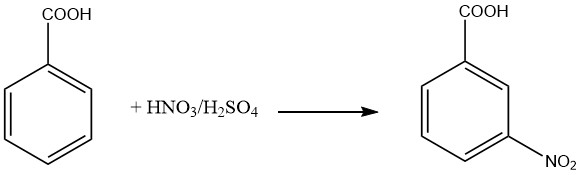
A higher yield of 3-nitrobenzaldehyde, a precursor to 3-Nitrobenzoic Acid, can be achieved by the controlled oxidation of benzaldehyde. 3-Nitrobenzoic Acid serves as an intermediate in the synthesis of 3-aminobenzoic acid and azo dyes. Its derivative, 4-chloro-3-nitrobenzoic acid, acts as an intermediate in the production of dyes.
4-nitrobenzoic acid or p-nitrobenzoic acid
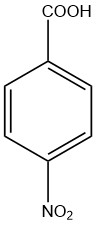
4-nitrobenzoic acid is a mono nitrobenzoic acid with the two groups in a para position with each other. It is also called parra-nitrobenzoic acid. It is a pale yellow solid that is insoluble in water and slightly soluble in methanol.
Physical properties of 4-nitrobenzoic acid
The physical properties of the 4-nitrobenzoic acid are the following:
- Molar mass = 167.12 g/mol
- Melting point = 240 °C
- Density = 1.61
- Solubility in water = 0.02 g/100 ml (at 15 °C)
- Solubility in methanol = 9.6 g/100 ml (at 10 °C)
- Solubility in benzene = 0.017 g/100 ml (at 12.5 °C)
- Dissociation constant, Ka (at 25 °C) = 3.93 × 10-4
- Decarboxylation temperature > 240 °C
Chemical reactions of 4-nitrobenzoic acid
4-nitrobenzoic acid undergoes the typical reaction of aromatic carboxylic acids and nitroaromatic compounds:
4-Aminobenzoic acid can be produced by the reduction of the nitro group into an amine.
The carboxylic group forms esters with alcohols and 4-nitrobenzoyl chloride with chlorinating agents wich is a starting material for procaine hydrochloride and folic acid.
At temperatures above 240 °C, 4-nitrobenzoic acid forms nitrobenze by decarboxylation.
The aromatic ring can undergo substitution reactions, but it is difficult due to the presence of deactivating groups.
Production of 4-nitrobenzoic acid
4-Nitrobenzoic Acid is produced commercially by the oxidation of 4-nitrotoluene with molecular oxygen. Oxidation with 15 % nitric acid at 175 °C produces the acid in 88.5 % yield.

Monsanto Chemicals Ltd. had developed a production method for 4-nitrobenzoic acid by oxidation of 4-nitroacetphenone with aqueous nitric acid in the presence of ammonium metavanadate as a catalyst under refluxing.
Eight moles of nitric acid are required to convert one mole of 4-nitroacetophenone at a temperature of 60 °C to the refluxing temperature of the mixture.
By cooling the reaction mixture, the crude 4-nitrobenzoic acid crystals form and are recovered therefrom by filtration, then purified by recrystallization from such solvents as benzene, ethanol, benzene-ethanol mixtures, hexane, etc.

An interesting method involves the nitration and subsequent oxidation of polystyrene. This method uses the steric hindrance of the polymer chain to improve the para to ortho ratio of the product.
References
- Benzoic Acid and Derivatives; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a03_555
- Preparation of 2-and 4-nitrobenzoic acid. – https://patents.google.com/patent/US2695311A/en
