Phthalic Acid: Properties, Production and Uses
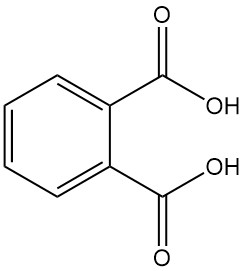
Phthalic acid, also known as o-phthalic acid or 1,2-benzenedicarboxylic acid, is an aromatic dicarboxylic acid with the chemical formula C8H6O4. It is a white, crystalline solid that is not important industrially. It is formed as a byproduct in the manufacture of phthalic anhydride.
Phthalic acid is found naturally in some plants, such as Papaver somniferum, Cocos nucifera, and other organisms and animals. It was first discovered in 1836 by the French chemist A. Laurent as a product of the oxidation of naphthalene.
Table of Contents
1. Physical Properties of Phthalic Acid
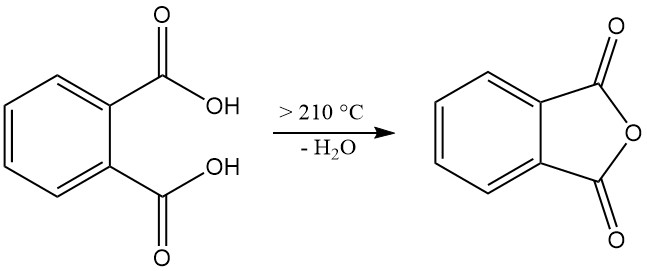
Phthalic acid [88-99-3] is a weak diprotic acid with dissociation constants pKa1 = 2.89 and pKa2 = 5.51. It forms colorless, monoclinic crystals with a melting point of 191 °C in a sealed tube that are converted into phthalic anhydride with the elimination of water at temperatures higher than 210 °C.
Phthalic acid is soluble in boiling water (100 °C), 18 g/100 mL, and is much less soluble in cold water (14 °C), 0.54 g/100 mL. It is soluble in ethanol (10 g/100 ml) and methanol (19.5 g/100 ml) and insoluble in chloroform and ether.
Some of the important physical properties of phthalic acid are listed in the following table.
| Property | Value |
|---|---|
| Formula | C8H6O4 |
| Molecular Weight | 166.14 g/mol |
| Melting Point | 191 °C (in sealed tube) Decomposition at 210 °C |
| Density at 15 °C | 1.593 g/cm3 |
| Vapor Density | 5.7 |
| Heat of Fusion | 315.3 J/g |
| Specific Heat of Solid (0–99 °C) | 1.214 J g-1 K-1 |
| Heat of Combustion | 19657.03 J/g |
| Heat of Formation | 43714.34 J/g |
| Heat of Solution at 25 °C | 123.55 J/g |
| Flash Point | 168 °C |
2. Reactions of Phthalic Acid
Phthalic acid undergoes typical chemical reactions of carboxylic acids and aromatic compounds.
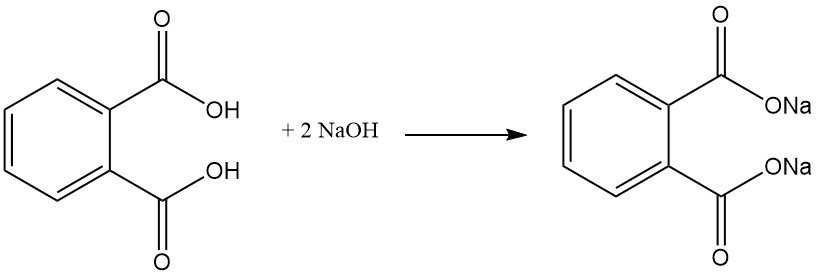
Phthalic acid is a weak diprotic acid that reacts with bases such as NaOH or KOH and metal oxides to form salts called phthalates. These salts can be soluble in water (salts formed with alkali metals (e.g., sodium, potassium)) or insoluble, like calcium and magnesium phthalates.
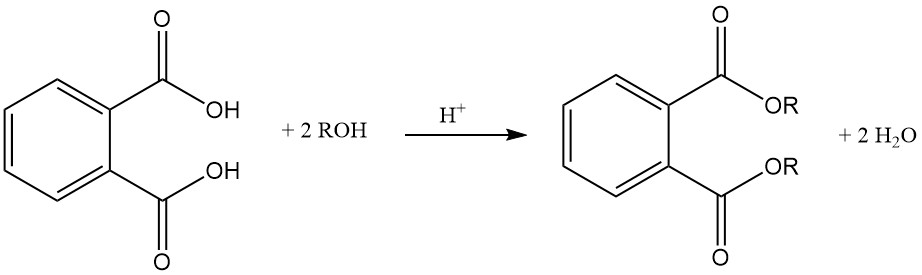
Phthalic acid reacts with alcohols in the presence of an acid catalyst (sulfuric acid) to form phthalate diesters. These esters are useful industrial chemicals with various applications.
When heated to 210 °C or above, phthalic acid undergoes dehydration to produce phthalic anhydride.
Phthalic acid can undergo aromatic substitution reactions; however, the presence of the two carboxylic acid groups desactivates it. Under specific and harsh conditions, further functionalization of the aromatic ring might be possible.
3. Production of Phthalic Acid
Phthalic acid is formed as a byproduct in the manufacture of phthalic anhydride by hydrolysis. Phthalic acid is not important in the industry. Instead, phthalic anhydride is produced industrially and then converted to phthalic acid if needed.
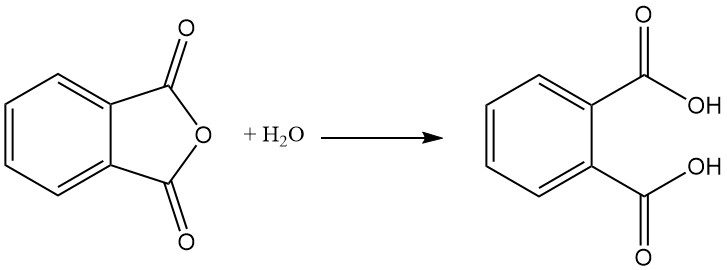
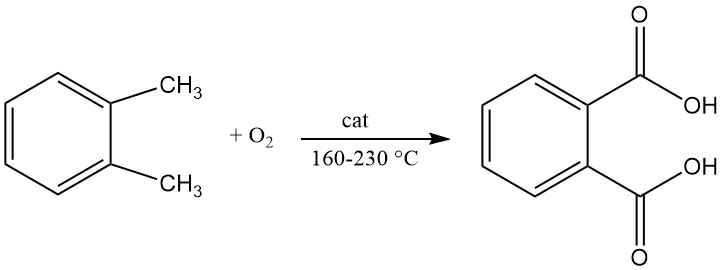
Phthalic anhydride is prepared by catalytic oxidation of naphthalene or ortho-xylene (o-xylene) at high temperatures and often uses a fixed-bed tubular reactor setup.
A patent in 1979 described the production of phthalic acid by continuous liquid oxidation of o-xylene with air in a stirred reactor. The reaction is carried out at a temperature between 160 °C and 200 °C and a pressure between 21 and 29 kg/cm2.
The catalyst used in this process contained a mixture of cobalt, manganese, and bromine and the water content in the reaction medium is between 0.2% and 7%.
The air is used as an oxidant, and the resultant liquid mixture contains 8% to 40% phthalic acid and 6% to 30% o-xylene. This liquid mixture is continuously fed, along with additional air, into a second stirred reactor.
The temperature in this second reactor is maintained between 210°C and 230°C. It’s important to note that fresh catalyst, containing at least 1 milligram atom of cobalt per mole of xylene processed (compared to the first stage), is added here.
This two-stage reaction yields a liquid effluent rich in phthalic acid (85% to 92%). However, it also contains water, impurities like benzoic acid, and higher boiling compounds.
4. Uses of Phthalic Acid
Phthalic acid is used as an analytical and laboratory reagent and to make medicines, dyes, phthalimide, anthranilic acid, phthalic acid esters, and synthetic perfumes.
Phthalic acid can be used as a precursor to phthalic anhydride, which is more valuable and is used in various industries like paints, plastics, textiles, and pharmaceuticals.
5. Toxicology and Hazards of Phthalic Acid
Phthalic acid is an intermediate that is produced and further used in industry under well-controlled conditions. Occupational exposure may occur but is expected to be low. The exposure of the general public to phthalic acid is low and negligible.
Despite the limited number of available toxicological studies, existing data suggests low toxicity for phthalic acid. Acute toxicity studies in mice (intraperitoneal injection) indicate a median lethal dose (LD50) of 550 mg/kg.
Following exposure, the majority of phthalic acid is excreted in the urine, either directly (as in dogs) or partially conjugated (as in rats and rabbits). A minor portion may undergo decarboxylation and be eliminated as benzoic acid.
References
- Phthalic Acid and Derivatives, Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a20_181.pub2
- https://patents.google.com/patent/US4215053A/en
- https://pubchem.ncbi.nlm.nih.gov/compound/Phthalic-acid
