Nitrilotriacetic Acid: Properties, Production, Uses and Toxicology
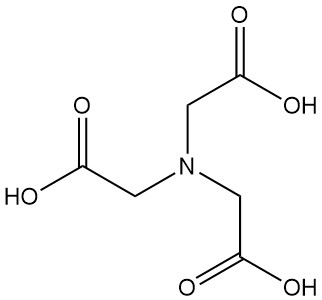
What is Nitrilotriacetic Acid?
Nitrilotriacetic acid, also known as NTA or N,N-bis(carboxymethyl)glycine, is a tricarboxylic acid with the formula C6H9NO6. It is a colorless solid that belongs to the family of aminopolycarboxylic acids and is one of the most important chelating agents, along with EDTA.
It was first synthesized in 1861 by Heintz by reacting ammonia with chloroacetic acid. Large-scale industrial production commenced in Ludwigshafen in 1936. Since then, nitrilotriacetic acid has established itself as a chelating agent used in various industrial processes.
Table of Contents
1. Physical Properties of Nitrilotriacetic Acid
Nitrilotriacetic acid is a triacid that forms colorless, needle-like crystals when crystallized from an aqueous solution. It is insoluble in water and most organic solvents; however, alkali metal nitrilotriacetates have high solubility in water. For example, trisodium nitrilotriacetate dissolves up to 640 g/L in water.
The most important physical properties of nitrilotriacetic acid are presented in the following table.
| Property | Value |
|---|---|
| CAS number | [139-13-9] |
| Chemical formula | C6H9NO6 |
| Molecular mass | 191.14 g/mol |
| Melting point | 242 °C (decomposition) |
| Water solubility | 0.13 g/100 g at 5 °C 0.13 g/100 g at 22.5 °C 0.95 g/100 g at 80 °C 3.3 g/100 g at 100 °C |
| pH of saturated solution | 2.3 |
| pK1 at 25 °C | 1.80 |
| pK2 at 25 °C | 2.48 |
| pK3 at 25 °C | 9.65 |
2. Chemical Properties of Nitrilotriacetic Acid
Nitrilotriacetic acid (NTA) is primarily characterized by the formation of water-soluble chelates with multivalent metal cations. Both carboxyl groups and tertiary nitrogen act as ligand sites. Unoccupied metal ion coordination positions can be occupied by water molecules.
While octahedral geometry (coordination number 6) often represents metal-NTA chelates, recent structural studies by X-ray diffraction indicate the potential for higher coordination numbers in certain metal complexes. For example, Ca2+ in CaNTA– complex displays a coordination number of 7.
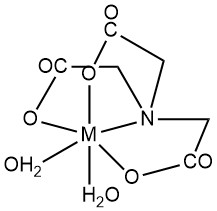
In general, metal complexes form in a 1:1 metal-to-NTA molar ratio, and excess NTA can lead to 1:2 complexes. Stability constants (K1 for 1:1, K2 for 1:2) quantify complex stability. The polarization of the H-O bond in the chelate gives a weak acid property to 1:1 complexes, characterized by the dissociation constant Kd.
K1 = [MNTA]/[M][NTA]
K2 = [MNTA2]/[M][NTA]2
Kd = [MNTAOH][H+]/[MNTA]
Where M is the multivalent metal ion, NTA represents the anion N(CH2COO–)3.
Table 2 lists some of the stability constants for NTA chelates.
| Metal ion | log K1 | log K2 | pKd |
|---|---|---|---|
| Al3+ | 11.4 | - | 5.09 |
| Ca2+ | 6.39 | 8.76 | - |
| Cd2+ | 9.78 | 14.39 | 11.25 |
| Co2+ | 10.38 | 14.33 | 10.80 |
| Cu2+ | 12.94 | 17.42 | 9.14 |
| Fe2+ | 8.33 | 12.80 | 10.60 |
| Fe3+ | 15.90 | 24.30 | 4.1-7.8 |
| Hg2+ | 14.60 | - | - |
| Mg2+ | 5.47 | - | - |
| Mn2+ | 7.46 | 10.94 | - |
| Ni2+ | 11.50 | 16.32 | 10.86 |
| Pb2+ | 11.34 | - | - |
| Zn2+ | 10.66 | 14.24 | 10.06 |
The complexation of metal-nitrilotriacetic acid competes with other reactions. Metal cations can form sparingly soluble precipitates with anions like carbonate, sulfide, sulfate, or oxalate. Moreover, hydrogen ions compete with metal cations for nitrilotriacetic trianion binding sites. Consequently, multiple equilibria control nitrilotriacetic acid systems.
Nitrilotriacetate chelates are stable in a wide pH range that depends on the chelate concentration and the excess complexing agent; for example, Ca2+ (pH 9–12), Mg2+ (pH 7–10), Cu2+ (pH 3–12), and Fe2+ (pH 1.5–3).
3. Production of Nitrilotriacetic Acid
The historical production of nitrilotriacetic acid from ammonia and chloroacetic acid is not used anymore. The oxidation of triethanolamine is not of industrial relevance.
Today, nitrilotriacetic acid is produced by either one-stage alkaline or two-stage acid cyanomethylation processes using ammonia (or ammonium sulfate), formaldehyde, and sodium cyanide (or hydrogen cyanide).
3.1. The alkaline process
The alkaline process is a long-established process where trisodium nitrilotriacetate is produced by the reaction of ammonia, formaldehyde, and sodium cyanide. The hydrolysis of the nitrilotriacetate yields nitrilotriacetate salt.
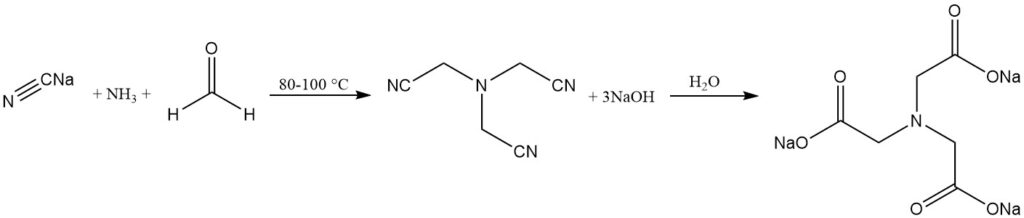
NH3 + 3 HCHO + 3 NaCN → N(CH2CN)3 + 3 NaOH
N(CH2CN)3 + 3 NaOH + 3 H2O → N(CH2COONa)3 + 3 NH3
This process can be conducted batchwise or continuously and takes place at 80–100 °C. At high pH (ca. 14), triscyanomethylamine is hydrolyzed in situ to trisodium nitrilotriacetate.
In an industrial continuous process, aqueous solution sodium cyanide is fed into a cascade reactor system along with formaldehyde solution. This method generates three-time excess ammonia, which needs to be limited to avoid the production of byproducts with low degrees of carboxymethylation, such as glycine and iminodiacetic acid.
The elimination of excess ammonia during the process is carried out by continuously distilling the ammonia with steam or air throughout the process; however, the formation of small amounts of undesirable products like glycolic acid and others mentioned before is inevitable.
The final solution is sold as a 40% solution, converted into powder, or acidified to form nitrilotriacetic acid.
3.2. The acid process
To limit the formation of byproducts in the alkaline process, some producers have constructed plants based on the acid process; however, this method demands stringent safety protocols due to the use of hydrogen cyanide, and there is also the corrosion problem.
First, ammonia and formaldehyde initially produce hexamethylenetetramine, which subsequently reacts with hydrogen cyanide in sulfuric acid to form triscyanomethylamine.
The solid triscyanomethylamine is insoluble in the reaction medium, so it is filtered off, washed, and then saponified with sodium hydroxide to yield a solution of trisodium nitrilotriacetate with minimal byproducts.
This solution is also sold as a 40% product or used in the production of powder or solid nitrilotriacetic acid.
Commercial nitrilotriacetic acid is sold in different trade names, including Dissolvine A grades (Akzo), Masquol NTA grades (Protex), Rexene NTA grades (Akzo), Trilon A grades (BASF), and Versene NTA grades (Dow).
4. Uses of Nitrilotriacetic Acid
The primary use of nitrilotriacetic acid and its salts is based on their ability to form complexes with metal ions. These complexation properties are used in diverse fields to sequester interfering metal ions, dissolve precipitates, modulate metal ion redox potentials, or create metal ion buffers.
4.1. Uses of Nitrilotriacetic Acid in Water Softening
Nitrilotriacetic acid effectively softens water by complexing calcium (Ca2+) and magnesium (Mg2+) ions within a neutral-to-alkaline pH range. The required nitrilotriacetic acid or trisodium nitrilotriacetate quantity for water softening varies based on water hardness.
Industries such as paper, textiles, soap, cosmetics, detergents, boiler feedwater treatment, and chemical processing (e.g., photography, electroplating) use nitrilotriacetic acid for water softening.
4.2. Uses of Nitrilotriacetic Acid as Phosphate Replacement in Detergents
Trisodium nitrilotriacetate is used as a substitute for pentasodium triphosphate in low-phosphate or phosphate-free detergents, often in conjunction with zeolites or polycarboxylates.
Unlike pentasodium triphosphate, trisodium nitrilotriacetate resists hydrolysis and remains fully effective after spray drying and during washing. While the equivalence ratio of trisodium nitrilotriacetate to pentasodium triphosphate is approximately 0.6 to 1, it does not compromise primary or secondary detergency.
4.3. Uses of Nitrilotriacetic Acid as Tetrapotassium Diphosphate Replacement in Cleansers
Trisodium nitrilotriacetate replaces tetrapotassium diphosphate in cleansers at a favorable 1:3 equivalence ratio, often eliminating the need for hydrotropes or solubilizers. Synergistic builder effects occur with partial phosphate substitution.
4.4. Uses of Nitrilotriacetic Acid in Heavy Metal Ion Masking
Although less effective than EDTA, nitrilotriacetic acid is used to mask heavy metal ions like Fe3+, Cu2+, and Mn2+. Industries such as soap manufacturing, textile bleaching, surface cleaning, and various chemical processes use it for this purpose.
4.5. Specialized Applications
Nitrilotriacetic acid is also used in specialized applications like rare earth element separation, complexometric titrations, and agricultural micronutrient formulation.
5. Toxicology of Nitrilotriacetic Acid
Nitrilotriacetic acid is highly biodegradable and readily mineralizes into inorganic end products without persistent organic metabolites.
Environmental studies, particularly following the introduction of detergents containing nitrilotriacetic acid, confirm its rapid biodegradation in wastewater treatment plants (>98%) and surface waters (half-life < 1 day). Minimal nitrilotriacetic acid traces are detectable in groundwater after limited infiltration.
While nitrilotriacetic acid’s complexing ability can potentially mobilize heavy metals, its rapid biodegradation minimizes this risk. Studies on wastewater treatment plants and river sediments indicate negligible heavy metal remobilization under realistic conditions.
Nitrilotriacetic acid demonstrates low acute toxicity to aquatic organisms, with LC50 values ranging from approximately 100 to over 10,000 mg/L, influenced by water hardness. Chronic toxicity studies on various organisms reveal no adverse effects below 1 mg/L, with significant toxicity thresholds at much higher concentrations.
The acute toxicity in mammals varies by species. The oral LD50 value for trisodium nitrilotriacetate in rodents is approximately 2000 mg/kg; this dose induces vomiting in dogs and monkeys.
Inhalation studies and skin/eye/respiratory tract irritation tests show minimal effects, with no sensitization or allergy indications. Nitrilotriacetic acid has no teratogenic effects, alone or in combination with heavy metals like cadmium and mercury. Furthermore, mutagenicity tests yield negative results.
Mammals do not metabolize nitrilotriacetic acid; instead, the kidneys excrete it unchanged. Renal tubule and ureter cells accumulate higher concentrations of nitrilotriacetic acid.
Subchronic and chronic studies link nitrilotriacetic acid to kidney toxicity due to electrolyte and iron metabolism disruptions. High doses can damage kidney, ureter, and bladder epithelial cells, potentially leading to the formation of tumors.
However, a significant threshold exists below tumor development, far exceeding the no adverse effect level (NOEL) of 14 mg/kg body weight per day.
Reference
- Nitrilotriacetic Acid; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a17_377.pub3
