Hydroxylamine: Properties, Reactions, Production and Uses

Hydroxylamine is an inorganic compound with the chemical formula NH2OH. It’s a white crystalline solid, but due to its hygroscopic nature, it is almost always found and used in aqueous solution.
Hydroxylamine, initially reported by LOSSEN in 1865, was later isolated as its free base in 1891 by LOBRY DE BRUYN. RASCHIG obtained a patent for the first industrial method of producing hydroxylammonium sulfate in Germany (1908) and the United States (1911).
The significance of hydroxylamine salts and their solutions lies in their substantial role as intermediates in various industrial processes, notably in the manufacturing of caprolactam. The global annual capacity for hydroxylamine production is estimated to reach 800,000 metric tons.
Table of Contents
1. Physical Properties of Hydroxylamine
Hydroxylamine, with the chemical formula NH2OH, has the characteristic of forming transparent crystals that are colorless and odorless.
It is highly soluble in water, as well as in methanol and ethanol. Several physical properties associated with hydroxylamine are as follows:
| Property | Value |
|---|---|
| Molar Mass | 33.03 g/mol |
| Melting Point (mp) | 32.05 °C |
| Boiling Point (bp) at 29 kPa | 56 °C |
| Vapor Pressure at 0 °C | 0.36 kPa |
| Vapor Pressure at 32 °C | 7.1 kPa |
| Enthalpy of Formation | -114 kJ/mol |
1.1. Hydroxylammonium Sulfate
The hydroxylammonium sulfate compound, also known as (NH3OH)2SO4 or (NH2OH)2·H2SO4, exists in a crystalline form that readily dissolves in water but exhibits only slight solubility in organic solvents.
The solid form of this compound has a density of 1.883 g/cm3, while the bulk density is approximately 1.10 g/cm3.
It is important to note that hydroxylammonium sulfate undergoes decomposition when exposed to temperatures above 120 °C.
Aqueous solutions of hydroxylammonium sulfate are clear and colorless. In a 1 wt % aqueous solution at 20 °C, the pH measures 3.6.
1.2. Hydroxylammonium Chloride
Crystalline hydroxylammonium chloride, also referred to as (NH3OH)Cl or NH2OH·HCl, is a hygroscopic salt that forms crystals. It exhibits high solubility in both water and methanol.
The addition of 0.5 wt % of pyrogenic silica effectively prevents caking, where the silica gel acts as an insoluble and inert component.
Hydroxylammonium chloride has a density of 1.676 g/cm3 and a bulk density of approximately 0.780 g/cm3.
Its decomposition occurs at temperatures above 120 °C. When dissolved in water, a 1 wt % aqueous solution of hydroxylammonium chloride at 20 °C has a pH value of 3.2.
2. Chemical Reactions of Hydroxylamine
Upon heating, hydroxylammonium salts undergo decomposition, and if local heating occurs, it can lead to runaway exothermic decomposition. The presence of heavy-metal impurities, particularly copper, copper-containing alloys, and copper salts, promotes the decomposition.
Decomposition of hydroxylammonium sulfate produces sulfur dioxide, dinitrogen monoxide (nitrous oxide), water, and ammonium sulfate. On the other hand, hydroxylammonium chloride decomposes into hydrogen chloride, nitrogen, water, and ammonium chloride.
When hydroxylammonium salts react with alkali, they yield hydroxylamine, which is easily decomposed. Reaction with nitrites results in decomposition to dinitrogen monoxide.
Aqueous solutions of hydroxylammonium salts exhibit acidity. Prolonged exposure to 80 °C leads to slow decomposition. Hydroxylamine and its salts act as radical traps in solution, leading to the formation of ammonia and nitrogen.
Oxidation of hydroxylamine, depending on the oxidant and reaction conditions, produces nitrogen compounds in various oxidation states.
Strong reducing agents such as zinc or iron powder yield ammonia. Many metal ions form complexes with hydroxylamine.
Hydroxylammonium salts can be transformed into hydroxylamine-O-sulfonic acid when reacted with oleum or chlorosulfonic acid. This acid serves as a suitable reagent for processes like amination, reductive deamination, hydroxymethylation, and the formation of heterocycles.
Hydroxylamine can react at both nitrogen and oxygen atoms. When aiming to prepare O-substituted hydroxylamines, protection of the nitrogen atom is necessary to avoid N-alkylation.
Hydroxylammonium compounds react with aldehydes and ketones to form oximes. β-Diketones and β-dialdehydes yield hydroxylamine isoxazoles.
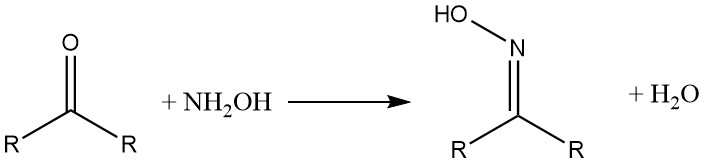
With carboxylic acids and their derivatives, hydroxylamine forms the corresponding hydroxamic acids.

Reaction with isocyanates and nitriles leads to the formation of N-hydroxyureas and amidoximes, respectively.

Hydroxylamine also reacts with olefinic double bonds:
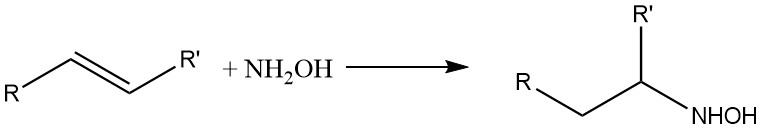
3. Production of Hydroxylamine
The industrial production of hydroxylamine involves the reduction of nitrogen’s higher oxidation states. For instance, nitric oxide or nitric acid can undergo catalytic hydrogenation to yield hydroxylamine.
The reduction of nitrous acid to hydroxylamine involves the use of sulfurous acid. In all these processes, the resulting product is an aqueous solution of a salt, NH2OH·HX (where HX represents an acid, such as sulfuric or phosphoric acid), or (NH3OH)X, rather than free hydroxylamine.
The acid hydrolysis of primary nitroalkanes, which concurrently produces the corresponding carboxylic acid, plays a minor role in the overall production of hydroxylamine.
3.1. Catalytic Hydrogenation of Nitric Oxide
To achieve high-purity nitric oxide, a two-step process is employed. Initially, ammonia and oxygen are reacted over a platinum-rhodium catalyst at temperatures exceeding 900 °C.
To ensure safety, the reaction gas is diluted with sufficient water or water vapor, bringing the mixture below the lower explosion limit. This reaction yields nitric oxide along with nitrogen dioxide and excess oxygen as byproducts.
The second step involves the hydrogenation of nitrogen dioxide and excess oxygen over a silver catalyst. The nitrogen dioxide is converted to nitric oxide, while the excess oxygen is transformed into water.
After condensation and simultaneous recovery of water vapor, the nitric oxide is purified using a washer.
It is then converted to hydroxylamine by reacting it with hydrogen below 50 °C over a partially poisoned platinum catalyst suspension in sulfuric acid. As byproducts, small amounts of ammonium sulfate and dinitrogen monoxide are produced.
The industrial process follows a continuous multistep approach, with extensive recirculation of exhaust gases.
The residual gas, containing hydrogen, nitrogen, nitric oxide, and dinitrogen monoxide, can be burned to recover steam. By carefully selecting the gas composition, explosive gas mixtures can be avoided during the synthesis of hydroxylamine.
Additionally, a modified process involves the catalytic hydrogenation of nitrogen monoxide, leading to the production of both cyclohexanone oxime and hydroxylamine. This method is known as acidic oximation.
3.2. Catalytic Hydrogenation of Nitrates
Hydroxylamine is commonly produced by the hydroxylamine-phosphate-oxime (HPO) process. This process involves the hydrogenation of an ammonium nitrate solution in the presence of phosphoric acid.
The HPO process is typically carried out alongside the synthesis of cyclohexanone oxime, which is used in the production of caprolactam.
3.3. The Raschig Process
The Raschig process, which is often modified, remains a significant method for the industrial production of hydroxylamine.
In the Raschig process, water, ammonia, and carbon dioxide undergo a reaction within an absorption column. This reaction leads to the formation of an ammonium carbonate solution, which at low temperatures reacts with nitrogen oxides to produce an alkaline solution of ammonium nitrite:
NH3 + H2O + CO2 → (NH4)2CO3
(NH4)2CO3 + NO + NO2 → 2 NH4NO2 + CO2
In the subsequent step, the ammonium nitrite is converted into ammonium hydroxylamine disulfonate by reacting it with sulfur dioxide:
NH4NO2 + 2 SO2 + NH3 + H2O → HO–N(SO3NH4)2
In this process, any excess ammonium hydroxylamine disulfonate is recycled. The solution containing ammonium hydroxylamine disulfonate is drawn off, and the salt is hydrolyzed and neutralized, resulting in the production of hydroxylammonium sulfate and ammonium sulfate as the final products.
HO–N(SO3NH4)2 + H2O → (NH3OH)2SO4 + (NH4)2SO4
3.4. Acid Cleavage of Nitroalkanes
The cleavage of mixtures containing nitropropane and nitromethane can be achieved by treating them with sulfuric acid at elevated temperatures. This reaction yields hydroxylammonium sulfate and the corresponding carboxylic acid:
Nitropropane + Nitromethane + Sulfuric Acid → Hydroxylammonium Sulfate + Carboxylic Acid
However, this process has been employed to a limited extent due to its high cost and the limited availability of the required raw materials.
4. Uses of Hydroxylamine
More than 95% of hydroxylamine production is dedicated to the manufacturing of cyclohexanone oxime or caprolactam.
Different producers utilize either hydrogenation or the Raschig process, with variations primarily observed in the amount of ammonium sulfate generated during oximation.
Hydroxylammonium salts serve as a stable form of hydroxylamine and find applications across various sectors in the chemical industry. Some notable uses include:
1. Chemical industry: Acting as a reactant for producing oximes as intermediates, as well as functioning as an oxidizing or reducing agent depending on the pH.
2. Paints and coatings: Utilized in the production of specialized oximes used as anticreaming agents.
3. Pharmaceuticals: Employed in the production of antibiotics, steroids, tranquilizers, spasmolytics, and antitubercular drugs.
4. Photography: Used as a stabilizer for developers and as an additive in color emulsions.
5. Rubber industry: Acts as a regulator in the polymerization of synthetic rubber, an antioxidant for natural rubber, and an accelerator in the vulcanization of synthetic rubber.
6. Soap: Serves as an auxiliary agent in refining fats for soap production.
7. Plastics: Functions as a regulator and inhibitor in various polymerization processes.
8. Metallurgy: Used as an additive for the surface treatment of steel, an auxiliary agent for metal extraction from electrolysis waste liquor, and for reclaiming spoil tips. Also serves as a flotation agent for copper ores.
9. Nuclear industry: Acts as an auxiliary agent for the separation of uranium and plutonium.
10. Textiles: Functions as an auxiliary agent in specific dyeing processes, a fixative for textile dyes, and a starting material for textile dyes based on isatin and isoxazole.
11. Crop protection: Serves as a starting material for the production of insecticides, herbicides, and plant growth regulators.
12. Food: Functions as an intermediate in the production of sweeteners.
These applications highlight the versatility and importance of hydroxylammonium salts across a wide range of industries.
5. Toxicology and Occupational Health
Hydroxylammonium compounds are considered harmful to health and can cause irritation. The LD50 (oral, rat) for hydroxylammonium compounds is approximately 600-640 mg/kg. In terms of aquatic toxicity, the LC50 (golden orfe, 48 hours) for hydroxylammonium sulfate ranges from 1 to 10 mg/L.
When in contact with the skin, hydroxylamine can cause slight to moderate irritation, and sensitization is possible. It also has a moderate to strong irritating effect on mucous membranes, such as the eyes.
Hydroxylamine can be absorbed through the skin and has the potential to form methemoglobin.
Studies examining the teratogenic effects of hydroxylamine (in rats) have shown no harmful effects on offspring. While hydroxylamine and its salts are not considered carcinogenic based on current data, they have been found to be mutagenic to lower organisms.
It is important to avoid contact with hydroxylammonium salts due to their irritant effects and the potential for dust to affect the skin, eyes, and throat. In case of contact with the skin or eyes, affected areas should be thoroughly rinsed with water.
Swallowing hydroxylammonium salts should be strictly avoided, and if necessary, a physician should be consulted. Hydroxylammonium salts should be labeled as dangerous materials.
Reference
- Hydroxylamine; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a13_527
FAQ: Hydroxylamine
Hydroxylamine can act as both an acid and a base, depending on the reaction and the environment it is in.
The conjugate acid of hydroxylamine is hydroxylammonium ion (NH3OH+)
Hydroxylamine is used in various applications, including the production of oximes as intermediates in the chemical industry, as an antioxidant and regulator in the rubber industry, in the synthesis of pharmaceuticals, dyes, and crop protection agents, and as a starting material for the production of caprolactam.
Hydroxylamine can be harmful and irritating to the skin, eyes, and mucous membranes. It should be handled with caution and proper safety measures. Contact with hydroxylamine salts should be avoided, and exposure should be minimized.
Hydroxylamine itself is not commonly used as a drug. However, it may be used as an intermediate in the synthesis of certain pharmaceuticals.
The nitrogen in hydroxylamine has a formal charge of 0 (neutral).
When hydroxylamine acts as a base, the nitrogen atom in hydroxylamine accepts a proton.
Ketone reacts with hydroxylamine to produce an oxime.
Hydroxylamine can be produced through various methods, such as the hydrogenation of nitric oxide or nitric acid, the Raschig process, or the hydrolysis and neutralization of ammonium nitrite. The specific method depends on the desired application and available technology.
