Fumaric Acid: Properties, Production and Uses
What is Fumaric Acid?
Fumaric acid, also known as trans-butenedioic acid, is a dicarboxylic acid with the formula C4H4O4. It is widely found in nature and appears as a white, crystalline solid with a fruit-like taste.
Fumaric acid was first isolated from fumitory (Fumaria officinalis) by Winckler in 1832. It was subsequently identified under various names, including boletic acid, glaucinium acid, lichen acid, and paramaleic acid.
Fumaric acid is now industrially produced, though it is less important compared to maleic anhydride.
Table of Contents
1. Physical Properties of Fumaric Acid
Fumaric acid crystallizes in the form of monoclinic prisms. It is soluble in ethanol and concentrated sulfuric acid, slightly soluble in ethyl ether and acetone, and insoluble in chloroform and benzene. A 0.05% aqueous solution of fumaric acid at 25 °C has a pH value of 3,0-3,2.
The important physical properties of fumaric acid are summarized in the table below.
| Property | Value |
|---|---|
| CAS number | [110-17-8] |
| Chemical formula | C4H4O4 |
| Molecular weight | 116.07 g/mol |
| Melting point | 286–287 °C (closed tube) |
| Sublimation temperature | 200 °C |
| Density (solid) | 1.635 g/cm3 |
| Heat of sublimation (92 °C) | 136.1 kJ/mol |
| pKa1 | 3.02 |
| pKa2 | 4.38 |
| Solubility in 100 g of water | 0.428 g (at 15.5 °C) |
| 9.97 g (at 100 °C) | |
| Flash Point | 273 °C (open cup) 230 °C (closed cup) |
| Autoignition Temperature | 375 °C (powder) |
2. Chemical Properties of Fumaric Acid
Fumaric acid sublimes without decomposition at or slightly above 200 °C, but when heated above 230 °C, it decomposes to form maleic anhydride, water, and a significant amount of residue. The presence of phosphorus pentoxide catalyzes maleic anhydride formation.
Fumaric acid is the trans-isomer of maleic acid and shares many chemical properties with it. However, reactions generally proceed slowly for fumaric acid, partially due to its lower water solubility.
Unlike maleic acid, fumaric acid can form an acid chloride. (for more details about the reactions, visit the article about maleic acid.)
3. Production of Fumaric Acid
Fumaric acid is primarily manufactured by the isomerization of maleic acid or maleic anhydride (Figure 1). These starting materials often come from washwater streams generated during the production of maleic anhydride or phthalic anhydride, with a minimum preferred maleic acid concentration of 30%.
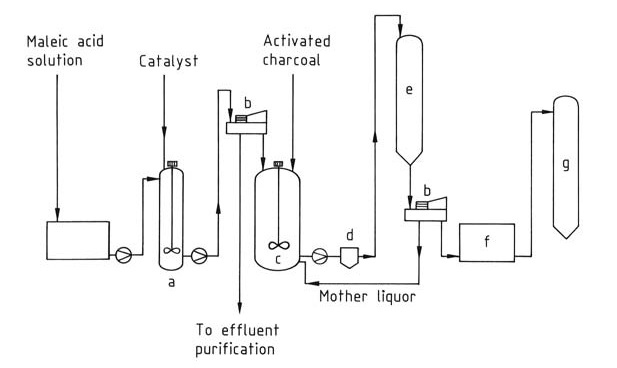
a) Isomerization vessel; b) Centrifuge; c) Dissolving tank; d) Filter; e) Crystallizer; f ) Dryer; g) Silo
The isomerization process converts maleic acid nearly quantitatively into the less soluble fumaric acid, which is then separated by filtration. This conversion can be achieved by thermal treatment or with the aid of catalysts. Several catalysts have been explored, including:
- Mineral acids (e.g., hydrochloric acid)
- Sulfur-based compounds like thiocyanates, thiazoles, thiosemicarbazides, and thioureas
- Bromine compounds combined with peroxides (e.g., persulfate)
Among these, thiourea is the most commonly used catalyst in the industry.
The wash water from the starting material can contain impurities that impact product quality and yield. This problem can be avoided by thermally pre-treating the wash water, adding urea alongside thiourea as a catalyst, or adding sulfites or sulfur dioxide gas, followed by mineral acids.
The obtained crude fumaric acid is purified by recrystallization from water and treatment with activated charcoal. These purification processes result in around 10% product loss.
Fumaric acid production is usually a batch-wise operation, although continuous processes exist. Choosing corrosion-resistant austenitic steel equipment is recommended, especially if hydrochloric acid is not involved in the process.
Disposal of the leftover mother liquor from the isomerization stage presents a challenge due to the presence of soluble impurities and the used catalyst. Combustion is a potential solution for this waste stream.
An alternative production method, employed in the United States, uses the fermentation of monosaccharides or starch with specific fungal strains such as Mucor, Aspergillus, and Rhizopus.
4. Uses of Fumaric Acid
Fumaric acid is a common food additive (E297) used as an acidulant or souring agent to enhance flavor in products like beverages, baked goods (especially rye and sourdough breads), and fillings. Additionally, it acts as a preservative by extending shelf life in items like wheat tortillas.
Fumaric acid has been used in oral medications and is being investigated for its potential role in treating psoriasis.
It is used as an intermediate in the production of various chemicals and in cosmetics (e.g., bath salts and denture cleaners).
Fumaric acid is often favored over maleic acid for the production of polyesters and copolymers. During the production process of these products, isomerization leads to a nearly equal mixture of trans and cis isomers of the dicarboxylic acids, regardless of the starting material (maleic or fumaric acid).
However, fumaric acid is preferred due to its safety profile (innocuous nature). In some cases, fumaric acid use can lead to specific effects, such as increased hardness in certain resins.
Fumaric acid (Fumaril) and iron (II) fumarate are used as specialized additives in animal feeds.
5. Toxicology of Fumaric Acid
Fumaric acid is a naturally occurring product with a favorable safety profile (nontoxic) based on various studies.
Animal studies indicate low acute toxicity. Oral LD50 values in rats exceed 10 g/kg body weight, and dermal application in rabbits up to 20 g/kg was not lethal.
Long-term administration of fumaric acid at 8 mg/kg body weight per day for a year in humans did not cause changes in blood, urine, or liver function.
No evidence of genotoxic effects (DNA damage), carcinogenicity (cancer-causing), or reproductive toxicity has been observed in experiments.
However, fumaric acid can act as a local irritant. It is classified as slightly irritating to the skin and moderately irritating to the eyes and mucous membranes upon contact.
References
- Maleic and Fumaric Acids; ULLMANN’S Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a16_053
- https://pubchem.ncbi.nlm.nih.gov/compound/Fumaric-Acid
